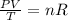Physics, 23.09.2019 11:30 caylah6101
According to the ideal gas law, increasing the volume of a closed reaction container decreases the thermal energy because the
a) r is divided.
b) temperature increases.
c) collision frequency remains the same.
d) pressure and collision frequency decrease.

Answers: 1


Another question on Physics

Physics, 21.06.2019 16:30
Matt is driving down 7th street. he drives 150 meters in 18 seconds. assuming he does not speed up or slow down, what is his speed?
Answers: 2

Physics, 22.06.2019 08:50
You are given a vector a = 125i and an unknown vector b that is perpendicular to a. the cross-product of these two vectors is a × b = 98k. what is the y-component of vector b?
Answers: 1

Physics, 22.06.2019 18:00
Air enters a gas turbine with two stages of compression and two stages of expansion at 100 kpa and 17°c. this system uses a regenerator as well as reheating and intercooling – the intercooler returns the air to the inlet temperature. the pressure ratio across each compressor is 4 ; 300 kj/kg of heat are added to the air in each combustion chamber; and the regenerator operates perfectly while increasing the temperature of the cold air by 20°c. determine the system’s thermal efficiency. assume isentropic operations for all compressor and the turbine stages and use constant specific heats at room temperature. (0.378)
Answers: 3

Physics, 22.06.2019 18:00
Atank is filled with an ideal gas at 400 k and pressure of 1.00 atm . part a the tank is heated until the pressure of the gas in the tank doubles. what is the temperature of the gas?
Answers: 3
You know the right answer?
According to the ideal gas law, increasing the volume of a closed reaction container decreases the t...
Questions

English, 03.07.2019 12:30

Mathematics, 03.07.2019 12:30

English, 03.07.2019 12:30

History, 03.07.2019 12:30


English, 03.07.2019 12:30

History, 03.07.2019 12:30


Health, 03.07.2019 12:30


Social Studies, 03.07.2019 12:30