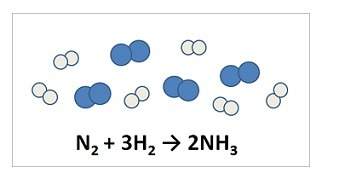
Nitrogen and hydrogen react to form ammonia. consider the mixture
of n2 (blue spheres) and h2...

Physics, 04.02.2020 18:47 mlittleduck6947
Nitrogen and hydrogen react to form ammonia. consider the mixture
of n2 (blue spheres) and h2 (gray spheres) in the illustration.
all but one statement regarding the reaction is true
a)nitrogen is in excess.
b)hydrogen is in excess.
c)this is a synthesis reaction.
d)hydrogen is the limiting reactant.

Answers: 3


Another question on Physics

Physics, 22.06.2019 17:30
Ethanol has a heat of vaporization of 38.56 kj/mol and a normal boiling point of 78.4 c. what is the vapor pressure of ethanol at 14 c?
Answers: 3


Physics, 23.06.2019 01:30
Amerry-go-round of radius r, shown in the figure, is rotating at constant angular speed. the friction in its bearings is so small that it can be ignored. a sandbag of mass m is dropped onto the merry-go-round, at a position designated by r. the sandbag does not slip or roll upon contact with the merry-go-round. how would you rank the following different combinations of m and r on the basis of the angular speed of the merry-go-round after the sandbag "sticks" to the merry-go-round from largest to smallest. a) m = 10kg, r = 0.25r b) m = 20kg, r = 0.25r c) m = 10kg, r = 0.50r d) m = 10kg, r = 1.0r e) m = 15kg, r = 0.75r f) m = 40kg, r = 0.25r
Answers: 1

Physics, 23.06.2019 09:00
Every winter i fly home to chicago. it takes 3 hours. what is my average speed?
Answers: 1
You know the right answer?
Questions

Physics, 20.04.2020 19:49


Mathematics, 20.04.2020 19:49

Mathematics, 20.04.2020 19:49

Mathematics, 20.04.2020 19:49



Mathematics, 20.04.2020 19:49

English, 20.04.2020 19:50



Mathematics, 20.04.2020 19:50







Mathematics, 20.04.2020 19:50




