Answers: 3


Another question on Physics

Physics, 21.06.2019 22:00
In a wind tunnel the speed changes as the cross sectional area of the tunnel changes. if the speed in a 6' x 6' square test section is 100 mph, what was the speed upstream of the test section where the tunnel measured 20' x 20'? use conservation of mass and assume incompressible flow. conservation of mass requires that as the flow moves through a path or a duct the product of the density, velocity and cross sectional area must remain constant; i.e., that ova-constant. a model is being tested in a wind tunnel at a speed of 100 mph if the flow in the test section is at sea level standard conditions, what is the pressure at the model's stagnation point? (a) the tunnel speed is being measured by a pitot-static tube connected to a u- tube manometer. what is the reading on that manometer in inches of water? (b) at one point on the model a pressure of 2058 psf is measured. what is the local airspeed at that point?
Answers: 2

Physics, 21.06.2019 22:30
A2 kg ball travellng to the right with a speed of 4 m/s collidees with a 5 kg ball traveling to the left with a speed of 3 m/s. take right to be the positive direction. what is the total momentum of the two balls before they collide? what is the total momentum of the two balls after they collide?
Answers: 1

Physics, 21.06.2019 23:00
How many dots must be added to the symbol to properly represent a standard nitrogen ion? a) 1 b) 3 c) 5 d) 8
Answers: 1

Physics, 22.06.2019 00:40
Electroplating is a way to coat a complex metal object with a very thin (and hence inexpensive) layer of a precious metal, such as silver or gold. in essence the metal object is made the cathode of an electrolytic cell in which the precious metal cations are dissolved in aqueous solution. suppose a current of 480.ma is passed through an electroplating cell with an aqueous solution of agno3 in the cathode compartment for 46.0 seconds. calculate the mass of pure silver deposited on a metal object made into the cathode of the cell. round your answer to 3 significant digits. also, be sure your answer contains a unit symbol. ×10μ
Answers: 3
You know the right answer?
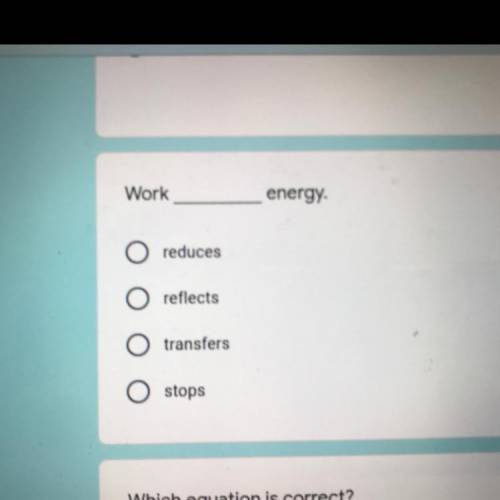
Reduce
Reflects
Transfers
Stops
...
Reflects
Transfers
Stops
...
Questions


Mathematics, 31.08.2019 02:00

History, 31.08.2019 02:00

Mathematics, 31.08.2019 02:00


Mathematics, 31.08.2019 02:00


English, 31.08.2019 02:00


Physics, 31.08.2019 02:00

History, 31.08.2019 02:00


Social Studies, 31.08.2019 02:00

Biology, 31.08.2019 02:00


History, 31.08.2019 02:00

Mathematics, 31.08.2019 02:00


Mathematics, 31.08.2019 02:00