
Which state of matter would be described as having low energy, with particles that are fixed in place and their only motion is vibrating? *
1 point
A. Liquid
B. Gas
C. Plasma
D. Solid
What is happening to the water molecules as it changes from ice to liquid water? *
1 point
A. They are losing energy, can’t move only vibrate
B. They are gaining energy, can move and slide past each other
C. They are moving at high speeds and are far from each other
D. They are gaining energy, moving slowly until they evaporate
Which state of matter has the highest energy level and is composed of freely moving charged particles? *
1 point
A. Gas
B. Liquid
C. Plasma
D. Solid
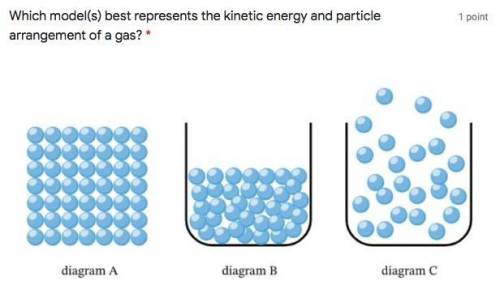
Which model(s) best represents the kinetic energy and particle arrangement of a gas? *
1 point
Captionless Image
A. Diagram A
B. Diagram B
C. Diagram C
D. Diagrams B and C
Which state of matter has the highest energy level and is composed of freely moving charged particles? *
1 point
A. Gas
B. Liquid
C. Plasma
D. Solid
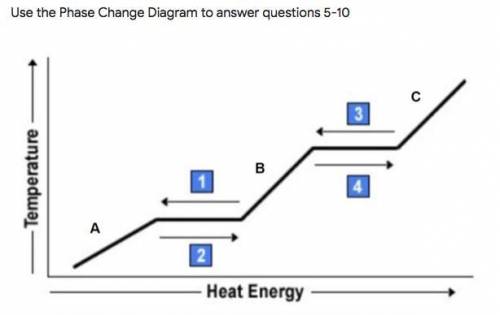
Use the Phase Change Diagram to answer questions 5-10
What phase change process is occurring at #2? *
1 point
A. Vaporization
B. Freezing
C. Melting
D. Condensation
What state of matter is represented by the letter A? *
1 point
A. Plasma
B. Liquid
C. Solid
D. Vapor
What phase change process is occurring at #3 and what is happening in terms of energy? *
2 points
A. Vaporization, energy is added
B. Vaporization, energy is removed
C. Condensation, energy added
D. Condensation, energy is removed
What state of matter is represented by the letter B and what phase change process is happening at #1? *
2 points
A. B is solid, and it is freezing as energy is removed
B. B is liquid, and it is evaporating as energy is removed
C. B is solid, and it is melting as energy is added
D. B is liquid, and it is freezing as energy is removed
What state of matter is represented by the letter C, and what would it change into if enough energy is added? *
1 point
A. Gas, it would change to vapor
B. Liquid, it would change to gas
C. Gas, it would change to plasma
D. Gas, it would change to liquid
What phase change process is occurring at #4? *
1 point
A. Condensation
B. Vaporization
C. Freezing
D. Melting
How does temperature affect the particle arrangement and state of matter of a substance? *
1 point
Captionless Image
A. As temperature increases the energy of particles increases and the substance changes from solid to liquid to gas
B. As temperature increases the energy of particles decreases and the substance changes from gas to liquid to solid
C. As temperature decreases the energy of particles increases and the substance changes from gas to liquid to solid
D. As temperature decreases the energy of particles decreases and the substance changes from solid to liquid to gas
What is the major difference between the solid, liquid, and gas phases of matter? *
2 points
Your answer
Submit.

Answers: 3


Another question on Physics

Physics, 21.06.2019 20:20
When many atoms are split in a chain reaction, a large explosion occurs. this is an example of what type of energy conservation
Answers: 2

Physics, 22.06.2019 13:00
The magnitude of the amount of energy released by burning a fuel source, measured in energy per unit mass, is called its fuel value. note that the fuel value is the negative of the isobaric specific heat of combustion for the fuel. if all the energy obtained from burning 1.23 pounds of butane with a fuel value of 10.85 kcal/g is used to heat 128.0 kg of water at an initial temperature of 18.3 °c, what is the final temperature? note that 1 lb = 453.6 g.
Answers: 3

Physics, 22.06.2019 17:10
Which statement best describes the superposition principle? a.) if two in-phase waves arrive simultaneously at a point, their amplitudes add up b.) if two out-of-phase waves arrive simultaneously at a point, their amplitudes add up c.) if two in-phase waves arrive at a point one after another, their amplitudes add up d.) if two out-of-phase waves arrive at a point one after another, their amplitudes adds up
Answers: 2

You know the right answer?
Which state of matter would be described as having low energy, with particles that are fixed in plac...
Questions



Mathematics, 15.07.2020 20:01






Mathematics, 15.07.2020 20:01



Geography, 15.07.2020 20:01


Mathematics, 15.07.2020 20:01


Mathematics, 15.07.2020 20:01


Mathematics, 15.07.2020 20:01


Mathematics, 15.07.2020 20:01



