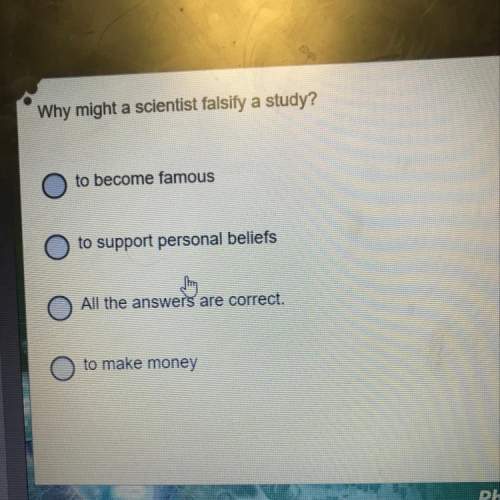
Well then i need some more i guess any suggestions
...

Physics, 04.02.2020 06:44 sherlyndaniel89
Well then i need some more i guess any suggestions

Answers: 2


Another question on Physics

Physics, 22.06.2019 21:20
Abeverage can is made of 3004-h19 aluminum alloy (elastic modulus 69 gpa, tensile yield strength 285 mpa, density 2.72 g/cm^3). the dimensions on the can are approximated as a thin-walled cylinder with a height of 4.83 inches, diameter of 2.60 inches. empty the can has a mass of 14.2 g. determine: a. the wall thickness of the cylinder b. assuming a pinned-pinned condition what is the critical load? c. assuming a fixed-fixed condition what is the critical load?
Answers: 1

Physics, 23.06.2019 02:30
Intro: a solid ball of radius rb has a uniform charge density ρ. part a: what is the magnitude of the electric field e(r) at a distance r> rb from the center of the ball? express your answer in terms of ρ, rb, r, and ϵ0. part b: what is the magnitude of the electric field e(r) at a distance r express your answer in terms of ρ, rb, r, and ϵ0. part c: let e(r) represent the electric field due to the charged ball throughout all of space. which of the following statements about the electric field are true? check all that apply. e(0) = 0 e(rb)=0 limr→∞e(r)=0 the maximum electric field occurs when r=0 the maximum electric field occurs when r=rb the maximum electric field occurs as r→∞.
Answers: 1

Physics, 23.06.2019 05:40
Whithin which type of system is neither the total mass nor the total energy conserved ? a. open b. connected c. isolated d. closed
Answers: 2

Physics, 23.06.2019 08:00
Which of the following is a false statement about dispersion forces? view available hint(s) which of the following is a false statement about dispersion forces? dispersion forces are the result of fluctuations in the electron distribution within molecules or atoms. dispersion forces are present in all atoms and molecules. dispersion forces result from the formation of instantaneous dipoles in a molecule or atom. dispersion forces always have a greater magnitude in molecules with a greater molar mass. dispersion force magnitude depends on the amount of surface area available for interactions.
Answers: 1
You know the right answer?
Questions

Biology, 09.09.2020 02:01

History, 09.09.2020 03:01





Mathematics, 09.09.2020 03:01


English, 09.09.2020 03:01











History, 09.09.2020 03:01