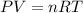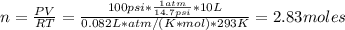Suppose you have a cylinder filled with diatomic oxygen (O2) and it is running low. The cylinder is shown above, is made of steel, and has a fixed volume of 10 L. You are asked to determine the number of O2 molecules that are left in the cylinder, so you take a measurement of the temperature to be 20℃. You then note that the pressure gauge reads 100 psi, which you checked at sea level in Bellingham, where the local pressure is one atm (14.7 psi). Calculate the number of O2 molecules left in the container.

Answers: 1


Another question on Physics



Physics, 22.06.2019 16:30
In a classical model of the hydrogen atom, the electron moves around the proton in a circular orbit of radius 0.053 nm. what is the electron's orbital frequency? what is the effective current of the electron?
Answers: 3

Physics, 22.06.2019 17:20
In a system with only a single force acting upon a body, what is the relationship between the change in kinetic energy and the work done by the force? answers: work is equal to the change in kinetic energy.work depends on the square of the change in potential energy.work is equal to the negative of the change in kinetic energy.work is equal to the square of the change in kinetic energy
Answers: 2
You know the right answer?
Suppose you have a cylinder filled with diatomic oxygen (O2) and it is running low. The cylinder is...
Questions




Health, 03.03.2021 04:10

History, 03.03.2021 04:10



English, 03.03.2021 04:10

History, 03.03.2021 04:10


Mathematics, 03.03.2021 04:10

Mathematics, 03.03.2021 04:10







History, 03.03.2021 04:10