What facts help to explain the pattern shown by the graph?
...

Physics, 22.02.2021 20:50 makiahlynn3642
What facts help to explain the pattern shown by the graph?
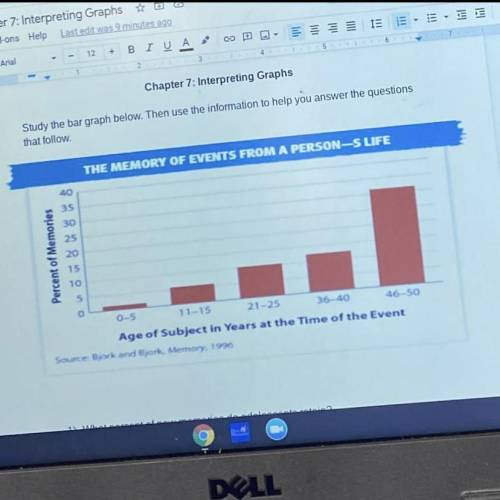

Answers: 2


Another question on Physics

Physics, 22.06.2019 00:20
Consider the particle-in-a-box problem in 1d. a particle with mass m is confined to move freely between two hard walls situated at x = 0 and x = l. the potential energy function is given as (a) describe the boundary conditions that must be satisfied by the wavefunctions ψ(x) (such as energy eigenfunctions). (b) solve the schr¨odinger’s equation and by using the boundary conditions of part (a) find all energy eigenfunctions, ψn(x), and the corresponding energies, en. (c) what are the allowed values of the quantum number n above? how did you decide on that? (d) what is the de broglie wavelength for the ground state? (e) sketch a plot of the lowest 3 levels’ wavefunctions (ψn(x) vs x). don’t forget to mark the positions of the walls on the graphs. (f) in a transition between the energy levels above, which transition produces the longest wavelength λ for the emitted photon? what is the corresponding wavele
Answers: 1

Physics, 22.06.2019 05:30
Will give brainliest! which statement best describes the difference between strong nuclear forces and weak nuclear forces? weak nuclear forces are involved when certain types of atoms break down. strong nuclear forces are responsible for holding atoms' nucleus together. weak nuclear forces hold bonds between atoms together. strong nuclear forces hold together the nucleus of an atom. strong nuclear bonds prevent atoms from falling apart. weak nuclear bonds prevent compounds from falling apart. strong nuclear forces are involved in breaking electrons from their shells. weak nuclear forces hold protons in the nucleus.
Answers: 3

Physics, 22.06.2019 12:30
Consider a hydrogen atom in the ground state. what is the energy of its electron? =e= jj now consider an excited‑state hydrogen atom. what is the energy of the electron in the =5n=5 level? =e5= j
Answers: 3

Physics, 22.06.2019 16:30
In a classical model of the hydrogen atom, the electron moves around the proton in a circular orbit of radius 0.053 nm. what is the electron's orbital frequency? what is the effective current of the electron?
Answers: 3
You know the right answer?
Questions


History, 17.01.2020 19:31


Physics, 17.01.2020 19:31



Mathematics, 17.01.2020 19:31







Mathematics, 17.01.2020 19:31

Biology, 17.01.2020 19:31


Spanish, 17.01.2020 19:31





