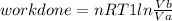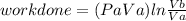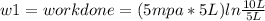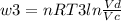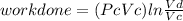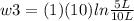Physics, 19.02.2021 17:00 bkimswift7
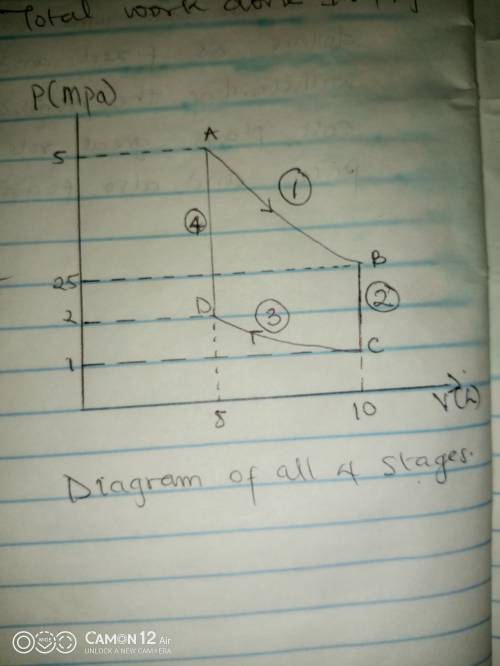
Thermodynamic Processes Two moles of a monatomic ideal gas at (5 MPa, 5 L) is expanded isothermally until the volume is doubled (step 1). Then it is cooled isochorically until the pressure is 1 MPa (step 2). The temperature drops in this process. The gas is now compressed isothermally until its volume is back to 5 L, but its pressure is now 2 MPa (step 3). Finally, the gas is heated isochorically to return to the initial state (step 4). (a) Draw the four processes in the pV plane. (b) Find the total work done by the gas.

Answers: 1


Another question on Physics

Physics, 21.06.2019 23:20
Imagine you had to physically add electrons, one at a time, to a previously neutral conductor. you add one electron very easily, but the second electron requires more work. in your initial post to the discussion, explain why this is. also, what happens to the work needed to add the third, fourth, fifth, and subsequent electrons
Answers: 1

Physics, 22.06.2019 10:30
Aparticle moves in the xy plane with constant acceleration. at time zero, the particle is at x = 6 m, y = 8.5 m, and has velocity ~vo = (9 m/s) ˆı + (−2.5 m/s) ˆ . the acceleration is given by ~a = (4.5 m/s 2 ) ˆı + (3 m/s 2 ) ˆ . what is the x component of velocity after 3.5 s? answer in units of m/s.
Answers: 1

Physics, 22.06.2019 15:30
What are the north and south poles of a solenoid change with?
Answers: 1

Physics, 22.06.2019 17:30
The items in the following list are all units of matter. which is the smallest unit that retains the properties of the matter? a.) atom b.) compound c.) electron d.) element
Answers: 1
You know the right answer?
Thermodynamic Processes
Two moles of a monatomic ideal gas at (5 MPa, 5 L) is expanded isothermally...
Questions




Mathematics, 23.07.2021 01:00



Mathematics, 23.07.2021 01:00





Mathematics, 23.07.2021 01:00

Mathematics, 23.07.2021 01:00

Mathematics, 23.07.2021 01:00


Mathematics, 23.07.2021 01:00


Computers and Technology, 23.07.2021 01:00