Physics, 18.02.2021 20:50 maddynichole2017
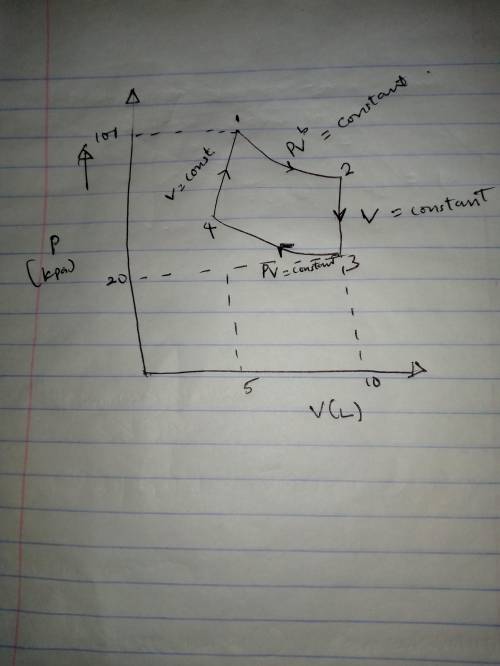
A mole of a monatomic ideal gas at point 1 (101 kPa, 5 L) is expanded adiabatically until the volume is doubled at point 2. Then it is cooled isochorically until the pressure is 20 kPa at point 3. The gas is now compressed isothermally until its volume is back to 5 L (point 4). Finally, the gas is heated isochorically to return to point 1.
a. Draw the four processes and label the points in the pV plane.
b. Calculate the work done going from 1 to 2.
c. Calculate the pressure and temperature at point 2.
d. Calculate the temperature at point 3.
e. Calculate the temperature and pressure and point 4.
f. Calculate the work done going from from 3 to 4.
g. Calculate the heat flow into the gas going from 3 to 4. g

Answers: 2


Another question on Physics

Physics, 22.06.2019 11:10
Which situation will produce the greatest change of momentum
Answers: 2

Physics, 22.06.2019 11:10
Avolcano erupts next to a grassland area in a valley. a. describe three ways in which material released by the volcano could impact the grassland area. b. describe three ways in which the grassland ecosystem could recover after a volcanic eruption.
Answers: 3

Physics, 22.06.2019 16:00
The energy produced as a result of this flow of electrons from atom to atom is called
Answers: 3

Physics, 22.06.2019 16:00
Which composition of water moves to begin a deepwater current?
Answers: 2
You know the right answer?
A mole of a monatomic ideal gas at point 1 (101 kPa, 5 L) is expanded adiabatically until the volume...
Questions


English, 23.06.2019 00:20





Biology, 23.06.2019 00:20

Geography, 23.06.2019 00:20

Biology, 23.06.2019 00:20

History, 23.06.2019 00:20




Social Studies, 23.06.2019 00:20

Mathematics, 23.06.2019 00:20





Mathematics, 23.06.2019 00:20