
Physics, 29.01.2021 06:40 gustavoroggero39
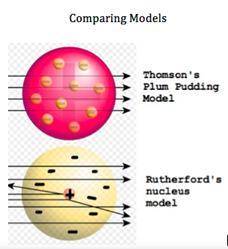
Atomic models have changed over the decades. Two early atomic models can be seen here. There is a dramatic change in the models, as Rutherford experimented with the cathode ray tube and charged particles. Differentiate between the two models by selecting the main difference between the models.
Question 5 options:
Rutherford's model shows negative charges dispersed throughout the atom
Rutherford's model shows negative particles orbiting the central nucleus
Rutherford's model shows the positive charge of an atom as a very small area
Thomson's model shows at sea of negative charged particles surrounding a small, positive area

Answers: 2


Another question on Physics

Physics, 22.06.2019 09:00
Sobre transformações cíclicas, são feitas algumas afirmações, determine quais estão corretas. i – a variação de energia interna em uma transformação cíclica é nula; ii – o trabalho, em uma transformação cíclica, é sempre positivo; iii – ao completar um ciclo, o gás tem a mesma temperatura com a qual começou a transformação; iv – uma transformação cíclica é, obrigatoriamente, composta de transformações conhecidas, como adiabática, isocórica, etc: a) i e iii b) i e ii c) ii e iii d) i e iv e) iii e iv
Answers: 1

Physics, 23.06.2019 00:00
What is the difference between mass density and weight density?
Answers: 1

Physics, 23.06.2019 00:00
You are a researcher for a golf club manufacturer. you are given two identical looking cubes of a metal alloy. you are informed that they are made of the exact same material, but one is crystalline, whereas the other is amorphous. it is your job to determine which one is amorphous this one is more stress-resistant and is useful in reinforcing golf clubs. which of the following is the best way to determine which is which? a. determine the density of each cube. the more dense one is the amorphous solid. b. melt both cubes and look for a broader range of melting temperatures. the one that melts over a broader range of temperatures is the amorphous solid. c. determine the density of each cube. the less dense cube is the amorphous solid. d. melt both cubes and measure the range of melting temperatures. the one that melts over a narrower range of temperatures is the amorphous solid.
Answers: 1

Physics, 23.06.2019 00:00
Which one of the following represents the reduced forms of the two major electron carriers? nadh and fadh2 nad+ and fadh2 nad+ and fad nadh and fad
Answers: 3
You know the right answer?
Atomic models have changed over the decades. Two early atomic models can be seen here. There is a dr...
Questions




Computers and Technology, 02.11.2019 06:31













Advanced Placement (AP), 02.11.2019 06:31





