
Physics, 28.12.2020 14:00 zeesharpe05
When 1.34 g Zn(s) reacts with 60.0 mL of 0.750 M HCl(aq), 3.14 kJ of heat are produced. Determine the enthalpy change per mole of zinc reacting for the reaction: Zn(s) +2HCl (aq) → ZnCl2 (aq) +H2 (g)

Answers: 1


Another question on Physics

Physics, 22.06.2019 04:00
When the force acting on an object points at least partially in the direction of the motion the work done is considered to be negative
Answers: 2

Physics, 22.06.2019 08:20
A3030 cmcm wrench is used to loosen a bolt with a force applied 0.30.3 mm from the bolt. it takes 6060 nn to loosen the bolt when the force is applied perpendicular to the wrench. how much force would it take if the force was applied at a 3030 degree angle from perpendicular? a 3030 cmcm wrench is used to loosen a bolt with a force applied 0.30.3 mm from the bolt. it takes 6060 nn to loosen the bolt when the force is applied perpendicular to the wrench. how much force would it take if the force was applied at a 3030 degree angle from perpendicular?
Answers: 3

Physics, 22.06.2019 16:00
The solid that is formed and usually sinks to the bottom of a solution is the
Answers: 2

You know the right answer?
When 1.34 g Zn(s) reacts with 60.0 mL of 0.750 M HCl(aq), 3.14 kJ of heat are produced. Determine th...
Questions

Biology, 28.11.2020 06:20

History, 28.11.2020 06:20

History, 28.11.2020 06:20

Chemistry, 28.11.2020 06:20

History, 28.11.2020 06:20

English, 28.11.2020 06:20




Computers and Technology, 28.11.2020 06:20


English, 28.11.2020 06:20

Social Studies, 28.11.2020 06:20

English, 28.11.2020 06:20


Physics, 28.11.2020 06:20




Mathematics, 28.11.2020 06:20

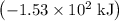 per mole
per mole 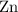 . (The negative value suggests the release of heat.) Assumption: the reaction was complete.
. (The negative value suggests the release of heat.) Assumption: the reaction was complete.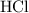 . Hence, either of the two species could be the limiting reactant. Calculating the number of moles of
. Hence, either of the two species could be the limiting reactant. Calculating the number of moles of 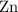 that was actually consumed requires finding the limiting reactant.
that was actually consumed requires finding the limiting reactant.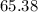 .
.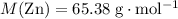 .
.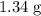 of
of 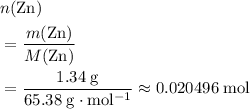 .
.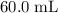 of
of 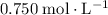 (
(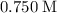 )
) 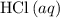 solution. Start by converting the unit of volume to liters (so as to match the unit of the concentration of this solution.)
solution. Start by converting the unit of volume to liters (so as to match the unit of the concentration of this solution.)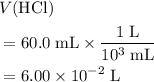 .
.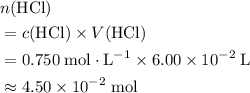 .
. .
.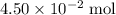 of
of 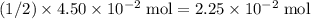
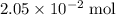 of
of 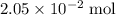 of
of 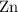 atoms would take part in this reaction.
atoms would take part in this reaction.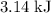 of heat through this reaction.
of heat through this reaction.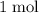 of
of 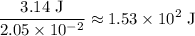 .
.

