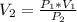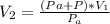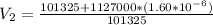Physics, 03.12.2020 17:00 juanitarodriguez
An air bubble released by a deep-water diver, 115 m below the surface of a lake, has a volume of 1.60 cm3. The surface of the lake is at sea level, and the density of the lake water can be approximated as that of pure water. As the bubble rises to the surface, the temperature of the water and the number of air molecules in the bubble can each be approximated as constant. Find the volume (in cm3) of the bubble just before it pops at the surface of the lake. ___ cm3.

Answers: 1


Another question on Physics

Physics, 21.06.2019 23:00
Asubmarine has a "crush depth" (that is, the depth at which water pressure will crush the submarine) of 250 m. what is the approximate pressure (water plus atmospheric) at this depth? (recall that the density of seawater is 1025 kg/m3, g = 9.81 m/s2, and 1 kg/(ms2) = 1 pa = 9.8692 10-6 atm.) a. 34.8 atm b. 24.8 atm c. 25.8 atm d. 7.8 atm
Answers: 2

Physics, 22.06.2019 02:10
Explain the consequences of this addiction to the brain tissue
Answers: 1

Physics, 22.06.2019 06:00
Why don't you see your reflection in water with waves or repples
Answers: 1

Physics, 22.06.2019 14:30
Suppose that 27 j of work is needed to stretch a spring from its natural length of 6 m to a length of 9 m. (a) how much work is needed to stretch the spring from 12 m to 14 m? j (b) how far beyond its natural length will a force of 78 n keep the spring stretched?
Answers: 2
You know the right answer?
An air bubble released by a deep-water diver, 115 m below the surface of a lake, has a volume of 1.6...
Questions

Social Studies, 05.05.2020 21:57

Chemistry, 05.05.2020 21:57


Mathematics, 05.05.2020 21:57



English, 05.05.2020 21:57



Arts, 05.05.2020 21:57

Mathematics, 05.05.2020 21:57



Mathematics, 05.05.2020 21:57




Mathematics, 05.05.2020 21:57


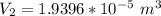
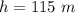
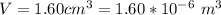
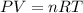
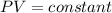
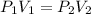
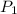 is the pressure of the bubble at the depth where it is released which i mathematically represented as
is the pressure of the bubble at the depth where it is released which i mathematically represented as 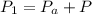
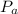 is the atmospheric pressure with value
is the atmospheric pressure with value 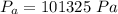
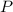 is the pressure due to the depth which is mathematically represented as
is the pressure due to the depth which is mathematically represented as 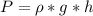
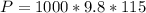
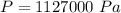
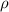 is the density of pure water with value
is the density of pure water with value 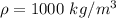
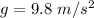
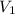 is the volume of the bubble at the depth where it is released
is the volume of the bubble at the depth where it is released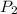 is the pressure of the bubble at the surface which is equivalent to the atmospheric temperature
is the pressure of the bubble at the surface which is equivalent to the atmospheric temperature 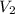 is the volume of the bubble at the surface
is the volume of the bubble at the surface