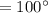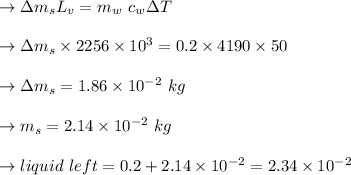Physics, 29.11.2020 15:10 kyasnead8189
In a perfectly insulated container of negligible mass, 4.00 × 10−2 kg of steam at 100◦C and atmospheric pressure is added to 0.200 kg of water at 50.0◦C. A) If no heat is lost to the surroundings, what is the final temperature of the system? B) At the final temperature, how many kilograms are there of steam and how many of liquid water?

Answers: 3


Another question on Physics


Physics, 22.06.2019 14:20
The energy released by a nuclear fusion reaction is produced when
Answers: 1

Physics, 22.06.2019 19:00
Friction removes energy from objects in motion. which statement best describes how this works? a) friction transforms ke into thermal energy b) friction transfers thermal energy to ke c) friction transforms te into pe d) friction transforms pe into ke e) friction transfers ke into pe
Answers: 1

Physics, 23.06.2019 13:40
What happens to light when it strikes the inside surface of a smooth, curved mirror?
Answers: 2
You know the right answer?
In a perfectly insulated container of negligible mass, 4.00 × 10−2 kg of steam at 100◦C and atmosphe...
Questions

English, 12.12.2021 01:10



Mathematics, 12.12.2021 01:10



Physics, 12.12.2021 01:10


Mathematics, 12.12.2021 01:10




Mathematics, 12.12.2021 01:10

Mathematics, 12.12.2021 01:10

Mathematics, 12.12.2021 01:10



Mathematics, 12.12.2021 01:10

Mathematics, 12.12.2021 01:10

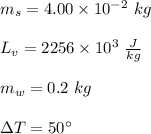
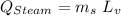
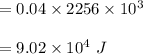
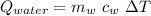
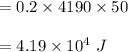
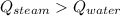 , that's why the final temperature is
, that's why the final temperature is