
Physics, 21.10.2020 16:01 caitlinsanders
HURRY PLS 30 MINS LEFT
A ball is rolling across the floor so that it is traveling along the positive x axis at 5.00 m/s. Due to the bend of the carpet fibers it experiences an acceleration of 1.25 m/s2 at an angle of -125.0o from the x axis.
A. What is the x component of the acceleration?
B. What is the y component of the acceleration?
C. What is the x component of the balls displacement after 3.00s?
D. What is the y component of the balls displacement after 3.00s?
E. What is the displacement of the ball over 3.00s?

Answers: 2


Another question on Physics

Physics, 22.06.2019 12:00
You have a resistor and a capacitor of unknown values. first, you charge the capacitor and discharge it through the resistor. by monitoring the capacitor voltage on an oscilloscope, you see that the voltage decays to half its initial value in 2.70 miss . you then use the resistor and capacitor to make a low-pass filter. what is the crossover frequency fc?
Answers: 2

Physics, 22.06.2019 13:20
It is reasonable to assume that the bulk modulus of blood is about the same as that of water (2.2 gpa). as one goes deeper and deeper in the ocean, the pressure increases by 10000 pa for every meter below the surface. if a diver goes down 80.0 m in the ocean, by how much does each cubic centimeter of her blood change in volume? give the answer in cubic centimeters (actually one cubic centimeter equals one milliliter).
Answers: 2

Physics, 23.06.2019 04:31
A90db sound wave strikes an eardrum whose area is 5.0×10^-5m^2. how much energy is absorbed by the eardrum per second
Answers: 1

Physics, 23.06.2019 08:00
Use henry's law and the solubilities given below to calculate the total volume of nitrogen and oxygen gas that should bubble out of 1.7 l of water upon warming from 25 ˚c to 50 ˚c. assume that the water is initially saturated with nitrogen and oxygen gas at 25 ˚c and a total pressure of 1.0 atm. assume that the gas bubbles out at a temperature of 50 ˚c. the solubility of oxygen gas at 50 ˚c is 27.8 mg/l at an oxygen pressure of 1.00 atm. the solubility of nitrogen gas at 50 ˚c is 14.6 mg/l at a nitrogen pressure of 1.00 atm. assume that the air above the water contains an oxygen partial pressure of 0.21 atm and a nitrogen partial pressure of 0.78 atm.
Answers: 2
You know the right answer?
HURRY PLS 30 MINS LEFT
A ball is rolling across the floor so that it is traveling along the positiv...
Questions

Mathematics, 08.12.2020 08:00



Mathematics, 08.12.2020 08:00


History, 08.12.2020 08:00

Mathematics, 08.12.2020 08:00

History, 08.12.2020 08:00

Mathematics, 08.12.2020 08:00

Biology, 08.12.2020 08:00



Mathematics, 08.12.2020 08:00

Business, 08.12.2020 08:00

Physics, 08.12.2020 08:00



History, 08.12.2020 08:00

Arts, 08.12.2020 08:00

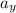 / a
/ a
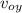 t + ½
t + ½ 

