
Physics, 15.10.2020 09:01 Onlyoneeniyaaa
A large research balloon containing 2.00 × 10^3 m^3 of helium gas at 1.00 atm and a temperature of 15.0°C rises rapidly from ground level to an altitude at which the atmospheric pressure is only 0.900 atm. Assume the helium behaves like an ideal gas and the balloon’s ascent is too rapid to permit much heat exchange with the surrounding air.
Required:
a. Calculate the volume of the gas at the higher altitude.
b. Calculate the temperature of the gas at the higher altitude.
c. What is the change in internal energy of the helium as the balloon rises to the higher altitude?

Answers: 3


Another question on Physics

Physics, 21.06.2019 20:30
When a book falls from a shelf to the ground without anyone pushing on it, no work is done on the book. true or false
Answers: 1

Physics, 22.06.2019 12:00
An architect plans to use solar energy to heat the next house he designs. what principle of absorption and infrared energy can be applied to the design of the new house? how could she apply to those principals?
Answers: 2

Physics, 22.06.2019 14:30
Slab pull” is a type of tectonic plate movement that occurs due to the forces of mantle convection and results in the subduction of the lithosphere true or false
Answers: 2

Physics, 22.06.2019 18:30
Which of the following is not a means to accelerating? question 4 options: a)increase speed b)remain still c)decrease speed d)change direction
Answers: 1
You know the right answer?
A large research balloon containing 2.00 × 10^3 m^3 of helium gas at 1.00 atm and a temperature of 1...
Questions

Chemistry, 14.01.2020 18:31

History, 14.01.2020 18:31

Mathematics, 14.01.2020 18:31

Biology, 14.01.2020 18:31

History, 14.01.2020 18:31

Mathematics, 14.01.2020 18:31





Computers and Technology, 14.01.2020 19:31

History, 14.01.2020 19:31

Mathematics, 14.01.2020 19:31

Mathematics, 14.01.2020 19:31

History, 14.01.2020 19:31

Mathematics, 14.01.2020 19:31

Mathematics, 14.01.2020 19:31

Biology, 14.01.2020 19:31


Mathematics, 14.01.2020 19:31

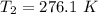
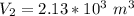
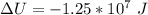
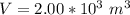
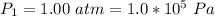
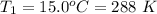
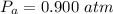
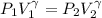
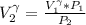
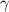 is a constant with value
is a constant with value 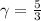 for an ideal gas
for an ideal gas 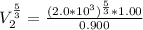
![V_2 = (\sqrt[5]{103.14641852} )^3](/tpl/images/0807/0306/7603b.png)
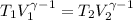
![T_2 = 288 * [\frac{2 * 10^{3}}{ 2.13 *10^{3}} ]^{ \frac{5}{3} -1 }](/tpl/images/0807/0306/ce7bb.png)
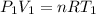
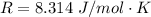
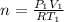

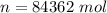
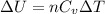
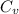 is the specific heats of gas at constant volume and the value is
is the specific heats of gas at constant volume and the value is 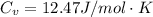
![\Delta U = 84362 * 12.47 * [T_2 - T_1 ]](/tpl/images/0807/0306/1f924.png)
![\Delta U = 84362 * 12.47 * [276.1 - 288 ]](/tpl/images/0807/0306/0b18f.png)


