Physics, 09.10.2020 01:01 cpcoolestkid4
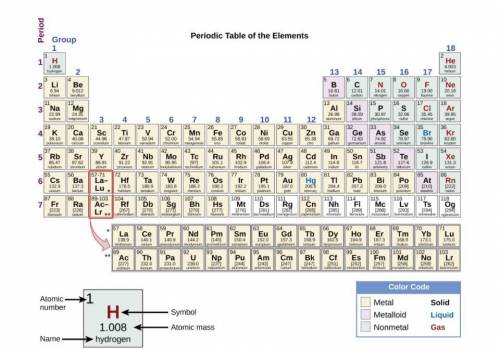
Elements are organized on the periodic table based on their properties. Which statement correctly predicts and explains the chemical reactivity of two metals?
Rubidium (Rb) is more reactive than strontium (Sr) because strontium atoms must lose more electrons.
Sodium (Na) is more reactive than magnesium (Mg) because sodium atoms must gain more electrons.
Calcium (Ca) is less reactive than potassium (K) because potassium atoms must lose more electrons.
Beryllium (Be) is less reactive than lithium (Li) because beryllium atoms must gain more electrons.

Answers: 3


Another question on Physics


Physics, 22.06.2019 18:00
Write a hypothesis about the effect of increasing resistance on the current in the circuit. use theformat and be sure to answer the lesson question. “how do changes in voltage or resistance affect current in an electric circuit? ”
Answers: 3

Physics, 23.06.2019 02:00
How many electrons does each element require in its valence shell to become stable?
Answers: 1

You know the right answer?
Elements are organized on the periodic table based on their properties. Which statement correctly pr...
Questions



History, 12.10.2020 08:01


English, 12.10.2020 08:01

Mathematics, 12.10.2020 08:01

Mathematics, 12.10.2020 08:01


Social Studies, 12.10.2020 08:01

Mathematics, 12.10.2020 08:01


History, 12.10.2020 08:01


Geography, 12.10.2020 08:01

Mathematics, 12.10.2020 08:01



Physics, 12.10.2020 08:01