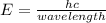Physics, 08.10.2020 03:01 natalieoppelt
In a test of a new UV-curable polymeric material (with thermal conductivity 0.15 W/(m K)) for dental prostheses, a 2.8 mm thick slab is subjected to monochromatic light with wavelength 313 nm, which is absorbed by the polymeric material according to the Beer Lambert Law of exponential attenuation. The material's surface is exposed to light at z = 0 , the rate at which light is absorbed (with dimensions of photons per unit volume per unit time) is 0 z I e γ γ − , where 0 I is the incident photon flux (with dimensions of photons per unit area per unit time), and 4 1 γ 3 10 m− − = × . (This approximation neglects the temporal decrease in absorption, among other things.) We also assume that all absorbed radiation is converted to thermal energy. (This neglects thermochemical change in the material.) If the two surfaces of the polymeric material (at z = 0 and z = 2.8 mm ) are held at 38°C, what is the largest allowable value of 0 I so that, at steady state, the maximum temperature in the polymeric material does not exceed 44°C

Answers: 3


Another question on Physics


Physics, 22.06.2019 11:10
Which situation will produce the greatest change of momentum
Answers: 2

Physics, 22.06.2019 12:10
Consider a one meter long horizontal pipe with a constant 100 cm^2 cross sectional area. water flows rightward into the pipe at x = 0 with flow velocity 02m/sec at every point within the pipe intake area. at x=1, the rightward flow rate is 0.192 m/sec. assume the water is a conserved quantity in the pipe, so there must be a leak (a sink) somewhere in the pipe. 1. compute net volumetric flow of the source if the system to be in equilibrium. 2. now assume the pipe in the problem has no leaks. compute the net volumetric rate of change for the system.
Answers: 3

Physics, 22.06.2019 13:00
The magnitude of the amount of energy released by burning a fuel source, measured in energy per unit mass, is called its fuel value. note that the fuel value is the negative of the isobaric specific heat of combustion for the fuel. if all the energy obtained from burning 1.23 pounds of butane with a fuel value of 10.85 kcal/g is used to heat 128.0 kg of water at an initial temperature of 18.3 °c, what is the final temperature? note that 1 lb = 453.6 g.
Answers: 3
You know the right answer?
In a test of a new UV-curable polymeric material (with thermal conductivity 0.15 W/(m K)) for dental...
Questions


History, 10.06.2020 13:57






History, 10.06.2020 13:57


History, 10.06.2020 13:57



Mathematics, 10.06.2020 13:57


English, 10.06.2020 13:57


Mathematics, 10.06.2020 13:57



History, 10.06.2020 13:57