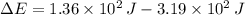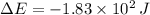A gas expands and does PV work on its surroundings equal to 319 J. At the same time, it absorbs 136 J of heat from the surroundings. Calculate the change in energy of the gas. Note: PV work means work done by a changing volume against constant pressure. Enter your answer in scientific notation.

Answers: 3


Another question on Physics

Physics, 22.06.2019 14:30
Suppose that 27 j of work is needed to stretch a spring from its natural length of 6 m to a length of 9 m. (a) how much work is needed to stretch the spring from 12 m to 14 m? j (b) how far beyond its natural length will a force of 78 n keep the spring stretched?
Answers: 2

Physics, 22.06.2019 17:40
Aball is thrown horizontally from a cliff at 8m/s .in what direction is the ball moving 2s later?
Answers: 1

Physics, 22.06.2019 18:50
8.29 two streams containing pyridine and acetic acid at 25°c are mixed and fed into a heat exchanger. due to the heat-of-mixing effect, it is desired to reduce the temperature after mixing to 25°c using a stream of chilled ethylene glycol as indicated in the diagram. calculate the mass flow rate of ethylene glycol needed. the heat capacity of ethylene glycol at these conditions is approximately 2.8 kj/(kg k), and the enthalpy change of mixing (δmixh) is given below.
Answers: 3

Physics, 22.06.2019 21:00
If the specific heat of a metal is 0.850 j/g °c, what is its atomic weight?
Answers: 2
You know the right answer?
A gas expands and does PV work on its surroundings equal to 319 J. At the same time, it absorbs 136...
Questions






Business, 01.07.2020 15:01


Mathematics, 01.07.2020 15:01


Health, 01.07.2020 15:01

Mathematics, 01.07.2020 15:01


English, 01.07.2020 15:01

Mathematics, 01.07.2020 15:01

Mathematics, 01.07.2020 15:01



Mathematics, 01.07.2020 15:01

Mathematics, 01.07.2020 15:01

Spanish, 01.07.2020 15:01

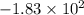 joules.
joules.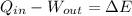
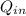 - Heat absorbed by the gas, measured in joules.
- Heat absorbed by the gas, measured in joules.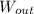 - Work done by the gas, measured in joules.
- Work done by the gas, measured in joules.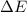 - Change in energy, measured in joules.
- Change in energy, measured in joules. 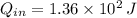 and
and 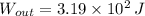 , the change in energy of the gas is:
, the change in energy of the gas is: