
Physics, 20.08.2020 05:01 miagiancarlo
.Study the scenario. A small container of water with a low temperature is poured into a large container of water with a higher temperature. Which choice correctly explains what happens to the thermal energy of these systems? A)The smaller container of water has more thermal energy than the larger container, and some of that energy is transferred to the warmer water in a process known as heating. B)The larger container of water has more thermal energy and some of that energy is transferred to the colder water in a process known as heating. C)The larger container of water has more heat and thermal energy than the smaller container. Some heat and thermal energy is transferred to the smaller container of water. D)The smaller container of water has more heat and thermal energy than the larger container. Some heat and thermal energy is transferred to the larger container of water.

Answers: 2


Another question on Physics

Physics, 22.06.2019 11:00
Consider a system to be two train cars traveling toward each other. what is the total momentum of the system before the train cars collide? kg • what must the total momentum of the system be after the train cars collide? kg •
Answers: 2

Physics, 22.06.2019 18:30
4. now look at the green lines you created by connecting the three boiling point data points and the three melting point data points. for each of these lines, describe any trends you see. 5. locate the elements on your periodic table that you circled in green on your graph. what term or description would you use to identify these elements with respect to the periodic table? 7. using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers.
Answers: 2

Physics, 22.06.2019 23:30
Newton's law of cooling states that the rate of change in the temperature t(t) of a body is proportional to the difference between the temperature of the medium m(t) and the temperature of the body. that is, startfraction dt over dt endfraction equals upper k left bracket upper m left parenthesis t right parenthesis minus upper t left parenthesis t right parenthesis right bracket , where k is a constant. let kequals0.04 left parenthesis min right parenthesis superscript negative 1 and the temperature of the medium be constant, m(t) font size decreased by 3 equivalent font size decreased by 3 294 kelvins. if the body is initially at 369 kelvins, use euler's method with hequals0.1 min to approximate the temperature of the body after (a) 30 minutes and (b) 60 minutes.
Answers: 2

Physics, 23.06.2019 07:00
A55-kg pilot flies a jet trainer in a half vertical loop of 1200-m radius so that the speed of the trainer decreases at a constant rate. knowing that the plane has a speed of 550 km/h at point a, and the pilot experiences weightlessness at point c (i.e., the normal force from the seat bottom is zero), determine (a) the deceleration of the plane, (b) the force exerted on her by the seat of the trainer when the trainer is at point b.
Answers: 2
You know the right answer?
.Study the scenario. A small container of water with a low temperature is poured into a large contai...
Questions

Biology, 28.08.2019 03:30

Mathematics, 28.08.2019 03:30

History, 28.08.2019 03:30







Computers and Technology, 28.08.2019 03:30

English, 28.08.2019 03:30

Geography, 28.08.2019 03:30






Mathematics, 28.08.2019 03:30


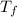 -T₀)
-T₀)



