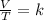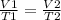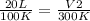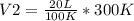Physics, 13.07.2020 21:01 lamontcarter
The Temperature of 20 liters of helium gas is increased from 100°K to 300°K. If the pressure was kept constant, the new volume will be how many liters? a) 6.7 b) 30 c) 120 e) None of these

Answers: 2


Another question on Physics

Physics, 21.06.2019 19:30
The 20@kg wheel has a radius of gyration about its center g of kg = 300 mm. when it is subjected to a couple moment of m = 50 n # m, it rolls without slipping. determine the angular velocity of the wheel after its mass center g has traveled through a distance of sg = 20 m, starting from rest.
Answers: 3

Physics, 22.06.2019 04:50
Unpolarized light whose intensity is 1.19 w/ is incident on a polarizer. (a) what is the intensity of the light leaving the polarizer? (b) if the analyzer is set at an angle of = 41.0∘ with respect to the polarizer, what is the intensity of the light that reaches the photocell?
Answers: 1

Physics, 22.06.2019 07:00
What type of relationship exists between the length of a wire and the resistance, if all other factors remain the same? o a. resistance is directly related to length. ob. resistance is directly related to the square of the length. c. resistance is inversely related to the length. d. resistance is inversely related to the square of the length. reset next
Answers: 1

Physics, 22.06.2019 10:40
When the magnetic domains in a material can be aligned, but eventually drift out of alignment, the material is
Answers: 2
You know the right answer?
The Temperature of 20 liters of helium gas is increased from 100°K to 300°K. If the pressure was kep...
Questions

Physics, 30.08.2021 23:20

Mathematics, 30.08.2021 23:20

History, 30.08.2021 23:20

Arts, 30.08.2021 23:20

Mathematics, 30.08.2021 23:20

Health, 30.08.2021 23:20

Mathematics, 30.08.2021 23:20

Mathematics, 30.08.2021 23:20



Mathematics, 30.08.2021 23:20




English, 30.08.2021 23:20

Mathematics, 30.08.2021 23:20

Biology, 30.08.2021 23:20