
Physics, 27.06.2020 20:01 ritaraum1802
An enclosed amount of nitrogen gas undergoes thermodynamic processes as follows: from an initial state A to a state B to C to D and back to A, as shown in the P-V diagram. Assume that the gas behaves ideally. What is the change in internal energy of the gas for the entire process, A-B-C-D-A? (pressure at B is 10kPa)
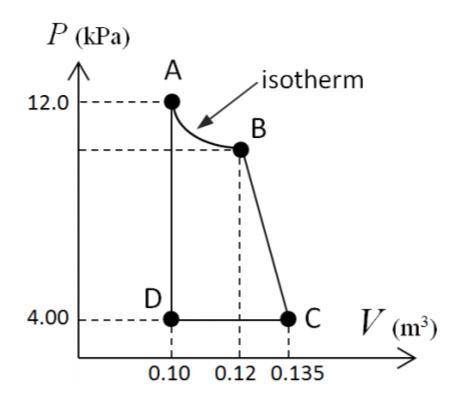

Answers: 2


Another question on Physics



Physics, 22.06.2019 19:30
The nuclear potential that binds protons and neutrons in the nucleus of an atom is often approximated by a square well. imagine a proton conned in an innite square well of length 105 nm, a typical nuclear diameter. calculate the wavelength and energy associated with the photon that is emitted when the proton undergoes a transition from the rst excited state (n 2) to the ground state (n 1). in what region of the electromagnetic spectrum does this wavelength belong?
Answers: 1

You know the right answer?
An enclosed amount of nitrogen gas undergoes thermodynamic processes as follows: from an initial sta...
Questions



History, 23.08.2019 06:50


Chemistry, 23.08.2019 06:50

Chemistry, 23.08.2019 06:50

Mathematics, 23.08.2019 06:50

World Languages, 23.08.2019 06:50

Mathematics, 23.08.2019 06:50


Social Studies, 23.08.2019 06:50

History, 23.08.2019 06:50

Physics, 23.08.2019 06:50






English, 23.08.2019 06:50

Mathematics, 23.08.2019 06:50

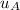 -
- 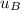 = 0
= 0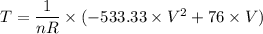
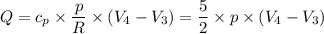
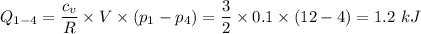
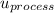 for the entire process is therefore;
for the entire process is therefore;

