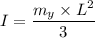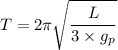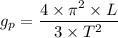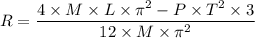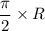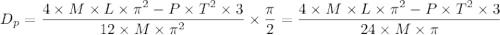Physics, 21.06.2020 01:57 tamarahawkins834
Because your spaceship has an engine failure, you crash-land with an emergency capsule at the equator of a nearby planet. The planet is very small and the surface is a desert with some stones and small rocks laying around. You need water to survive. However, water is only available at the poles of the planet. You find the following items in your emergency capsule: • Stopwatch • Electronic Scale • 2 yardstick • 1 Litre Oil • Measuring Cup Describe an experiment to determine your distance to the poles by using the available items. Hint: As the planet is very small, you can assume the same density everywhere.

Answers: 1


Another question on Physics

Physics, 21.06.2019 22:00
Identify the forces that contribute to the centripetal force on the object in each of the following examples: a. a bicyclist moving around a flat, circular track b. a bicycle moving around a flat, circular track c. a racecar turning a corner on a steeply banked curve
Answers: 1

Physics, 22.06.2019 10:30
An insulated 40 ft^3 rigid tank contains air at 50 psia and 120°f. a valve connected to the tank is now opened, and air is allowed to escape until the pressure inside drops to 25 psia. the air temperature during this process is kept constant by an electric resistance heater placed in the tank. determine the electrical work done during this process.
Answers: 2

Physics, 22.06.2019 18:00
Air enters a gas turbine with two stages of compression and two stages of expansion at 100 kpa and 17°c. this system uses a regenerator as well as reheating and intercooling – the intercooler returns the air to the inlet temperature. the pressure ratio across each compressor is 4 ; 300 kj/kg of heat are added to the air in each combustion chamber; and the regenerator operates perfectly while increasing the temperature of the cold air by 20°c. determine the system’s thermal efficiency. assume isentropic operations for all compressor and the turbine stages and use constant specific heats at room temperature. (0.378)
Answers: 3

Physics, 22.06.2019 19:30
A47.2 g block of copper whose temperature is 480 k is placed in an insulating box with a 91.8 g block of lead whose temperature is 200 k. (a) what is the equilibrium temperature of the two-block system? (b) what is the change in the internal energy of the two-block system between the initial state and the equilibrium state? (c) what is the change in the entropy of the two-block system? the heat capacities of copper and lead are 386 j/kg·k and 128 j/kg·k, respectively.
Answers: 1
You know the right answer?
Because your spaceship has an engine failure, you crash-land with an emergency capsule at the equato...
Questions

English, 28.10.2019 22:31

Mathematics, 28.10.2019 22:31

Social Studies, 28.10.2019 22:31






Mathematics, 28.10.2019 22:31


Computers and Technology, 28.10.2019 22:31




English, 28.10.2019 22:31




Mathematics, 28.10.2019 22:31

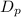 , is given as follows;
, is given as follows;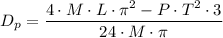
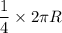
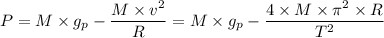
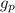 = Acceleration due to gravity on the planet
= Acceleration due to gravity on the planet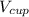 , of the oil equivalent to the mass of the cup from which the density of the oil is calculated as follows;
, of the oil equivalent to the mass of the cup from which the density of the oil is calculated as follows;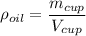
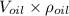
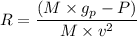
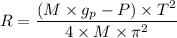
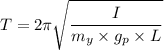
 = Mass of the yardstick
= Mass of the yardstick