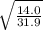Physics, 18.06.2020 18:57 SupremeNerdx
Fill in the blanks for the following: Arigid container of volume 100.0 Liters contains Oxygen gas. It is at room temperature (293 Kelvin), and is at atmospheric pressure (absolute pressure, meaning the gauge pressure is zero). Therefore, the number of moles of Oxygen molecules . Also, the rms-velocity inside is of these Oxygen molecules is most nearly , which is the rms-speed of the Nitrogen molecules just outside the container (the rigid container and its surroundings are in thermal equilibrium).

Answers: 3


Another question on Physics

Physics, 21.06.2019 18:20
What color would an object that reflects green and blue light appear to be in white light? a. blue b. cyan c. magenta d. black
Answers: 1

Physics, 21.06.2019 22:00
Atank holds 1000 gallons of water, which drains from the bottom of the tank in half an hour. the values in the table show the volume v of water remaining in the tank (in gallons) after t minutes.t(min) 5 10 15 20 25 30v(gal) 694 444 250 111 28 0(a) if p is the point (15, 250) on the graph of v, find the slopes of the secant lines pq when q is the point on the graph with t = 5, 10, 20, 25, and 30.(b) estimate the slope of the tangent line at p by averaging the slopes of two secant lines.(c) use a graph of the function to estimate the slope of the tangent line at p. (this slope represents the rate at which the water is flowing from the tank after 15 minutes.)show all steps.
Answers: 2

Physics, 22.06.2019 08:00
Why is it important always to use horizontal bars in unit fractions when performing unit conversions?
Answers: 3

Physics, 22.06.2019 09:30
For this car, predict the shape of a graph that shows distance (x) versus time (t). note that time is the independent variable and will be on the bottom axis. need asap
Answers: 1
You know the right answer?
Fill in the blanks for the following:
Arigid container of volume 100.0 Liters contains Oxygen gas....
Questions

Arts, 31.07.2019 10:00

Mathematics, 31.07.2019 10:00


History, 31.07.2019 10:00



Health, 31.07.2019 10:00


History, 31.07.2019 10:00





Social Studies, 31.07.2019 10:00


Biology, 31.07.2019 10:00

Advanced Placement (AP), 31.07.2019 10:00


Biology, 31.07.2019 10:00

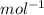

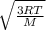
 = 478.6 m/s
= 478.6 m/s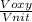 =
= 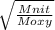
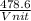 =
=