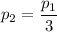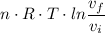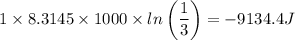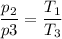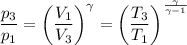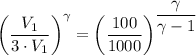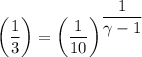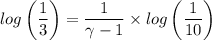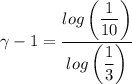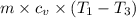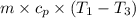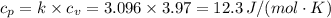Physics, 17.06.2020 05:57 tanyalynn12345
An ideal diatomic gas undergoes a cyclic process. In the first step, the gas undergoes an isothermal expansion from V1 to 3.00 V1. In the second step of the process the gas undergoes an isovolumetric decrease in pressure. In the third step the gas undergoes an adiabatic compression from 3.00 V1 back to V1 completing the cycle.
Required:
a. Sketch the cycle.
b. In terms of P., V. and T., determine P2, P3, T3.
c. In terms of P., V. To determine W, Q and ΔE int for each step. Take T, to be between 100K and 1000K

Answers: 3


Another question on Physics

Physics, 22.06.2019 04:00
10 newton object is placed 3 meters from the fulcrum. at what distance on the other side would you need to place a 15 newton object to balance the lever? show your work!
Answers: 2

Physics, 22.06.2019 06:20
Oxygen undergoes an isentropic expansion from 120 degree c and 280 kpa absolute to 140 kpa absolute. determine the final temperature of the oxygen and the amount of work per kg of o_2 produced in the process if it is adiabatic.
Answers: 1

Physics, 22.06.2019 10:30
If y gets smaller as x gets bigger and y have an relationship?
Answers: 1

Physics, 22.06.2019 18:30
Suppose you plot the distance traveled by an object at various times and you discover that the graph is not a straight line. what does this indicate about the object's acceleration?
Answers: 3
You know the right answer?
An ideal diatomic gas undergoes a cyclic process. In the first step, the gas undergoes an isothermal...
Questions


Arts, 03.02.2021 01:30



English, 03.02.2021 01:30


Mathematics, 03.02.2021 01:30

Mathematics, 03.02.2021 01:30

Mathematics, 03.02.2021 01:30

History, 03.02.2021 01:30


Mathematics, 03.02.2021 01:30

Mathematics, 03.02.2021 01:30



History, 03.02.2021 01:30


Mathematics, 03.02.2021 01:30


Mathematics, 03.02.2021 01:30