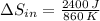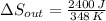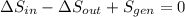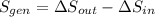Physics, 12.06.2020 01:57 itisonlyluis
When an aluminum bar is connected between a hot reservoir at 860 K and a cold reservoir at 348 K, 2.40 kJ of energy is transferred by heat from the hot reservoir to the cold reservoir
(a) In this irreversible process, calculate the change in entropy of the hot reservoir.
J/K
(b) In this irreversible process, calculate the change in entropy of the cold reservoir.
J/K
(c) In this irreversible process, calculate the change in entropy of the Universe, neglecting any change in entropy of the aluminum rod.
J/K
(d) Mathematically, why did the result for the Universe in part (c) have to be positive?

Answers: 3


Another question on Physics

Physics, 21.06.2019 23:00
Atrain departs from its station at a constant acceleration of 5 m/s. what is the speed of the train at the end of 20s?
Answers: 1

Physics, 22.06.2019 14:30
Which of these is constant for all types of electromagnetic radiation in a vacuum? select one: a. wavelength b. frequency c. photon energy d. amplitude e. velocity
Answers: 3

Physics, 23.06.2019 07:30
Why would an orange roll of your cafeteria tray if you stopped suddenly
Answers: 1

Physics, 23.06.2019 11:20
Answer fast for brainliest an optical telescope is an instrument that collects and reflects light from distant objects focuses light from very small objects stores heat from distant objects focuses light from distant objects
Answers: 2
You know the right answer?
When an aluminum bar is connected between a hot reservoir at 860 K and a cold reservoir at 348 K, 2....
Questions

Mathematics, 06.12.2020 05:40




History, 06.12.2020 05:40

Spanish, 06.12.2020 05:40

Social Studies, 06.12.2020 05:40

English, 06.12.2020 05:40

Mathematics, 06.12.2020 05:40

Mathematics, 06.12.2020 05:40


Arts, 06.12.2020 05:40

Computers and Technology, 06.12.2020 05:40


Mathematics, 06.12.2020 05:40

Social Studies, 06.12.2020 05:40

English, 06.12.2020 05:40



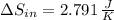 , b)
, b) 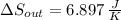 , c)
, c) 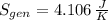 , d) Due to irreversibilities due to temperature differences.
, d) Due to irreversibilities due to temperature differences.