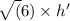Learning Goal:To understand and be able to use the rules for determining allowable orbital angular momentum states. Several numbers are necessary to describe the states available to an electron in the hydrogen atom. The principal quantum number ndetermines the energy of the electron. The orbital quantum number l determines the total angular momentum of the electron, and the magnetic quantum number mldetermines the component of the angular momentum parallel to a specific axis, usually the z axis. For a given principal quantum number n, the orbital quantum number can take integer values ranging from zero to n−1. For a given orbital quantum number l, the magnetic quantum number can take integer values from −l to l. A fourth number, the spin ms, is important for interactions with magnetic fields and counting states. The spin can be either +1/2 or −1/2, independent of the values of the other quantum numbers. The energy of an electron in hydrogen is related to the principal quantum number by En=(−13.60eV)/n2. The orbital angular momentum is related to the orbital quantum number by L=ℏl(l+1)−−−−−−√, and the orbital angular momentum in the z direction is related to the magnetic quantum number by Lz=mlℏ.
A. How many different values of I are possible for an electron with principal quantum number n = 5?
B. How many values of mi are possible for an electron with orbital quantum number I = 3? Express your answer as an integer.
C. The quantum state of a particle can be specified by giving a complete set of quantum numbers (n, l, m1, ms). How many different quantum states are possible if the principal quantum number is n = 3? To find the total number of allowed states, first write down the allowed orbital quantum numbers I, and then write down the number of allowed values of mi for each orbital quantum number. Sum these quantities, and then multiply by 2 to account for the two possible orientations of spin.
D. Is the state n = 3,1 = 3, m1 = -2, ms = 1/2 an allowable state? If not, why not?
a. Yes it is an allowable state.
b. No: The magnetic quantum number cannot be negative.
c. No: The magnetic quantum number must equal the orbital quantum number.
d. No: The orbital quantum number cannot equal the principal quantum number.
e. No: The magnetic quantum number must equal the principal quantum number.
E. What is the maximum angular momentum L max that an electron with principal quantum number n = 3 can have? Express your answer in units of h. (You don't need to enter the h, it is in the units field for you.)

Answers: 3


Another question on Physics

Physics, 21.06.2019 20:00
The first law of thermodynamics states that heat added to a system is neither created nor destroyed but is transformed ⇒ as it changes into other forms of energy.
Answers: 1

Physics, 22.06.2019 08:30
Hey student studies gravity using objects that have the same mass which two objects have the greatest gravitational force acting between them a. 100kg 1.0m 100kg b. 100kg 2.0m 100kg c. 100kg 2.0m 100kg big d. 100kg big 3.0m 100kg big
Answers: 1

Physics, 22.06.2019 15:20
What component of earth’s atmosphere exists entirely as a result of photosynthesis?
Answers: 2

Physics, 22.06.2019 17:30
Describe cytokinesis in plants and animals. in animals: in plants.
Answers: 2
You know the right answer?
Learning Goal:To understand and be able to use the rules for determining allowable orbital angular m...
Questions



Mathematics, 23.07.2019 17:00





Mathematics, 23.07.2019 17:00


History, 23.07.2019 17:00

Chemistry, 23.07.2019 17:00

History, 23.07.2019 17:00

History, 23.07.2019 17:00

Biology, 23.07.2019 17:00