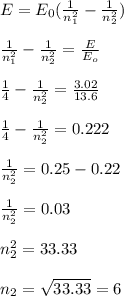Physics, 29.05.2020 00:03 kutemigos9211
MODERN PHYSICS
A photon emitted from an excited hydrogen atom has an energy of 3.02 electron volts. Which electron energy-level transition would produce this photon?
A. n=1 to n=6
B. n=2 to n=6
C. n=6 to n=1
D. n=6 to n=2
I chose B but the correct answer is D can someone tell me why? And what's the difference?

Answers: 1


Another question on Physics

Physics, 22.06.2019 04:20
Astone is thrown into a pond. what happens to the amplitude of the resulting waves as they get farther from the point where the stone hit the water? explain.
Answers: 3

Physics, 22.06.2019 08:30
What object a collides with object b and bounces back its final momentum is?
Answers: 1

Physics, 22.06.2019 14:30
What conclusion can be made based on the temperature of soil when the light hits the soil at 0°, 45°, and 90° angles in section 2 of the experiment? did your results support your hypothesis? why or why not?
Answers: 1

Physics, 22.06.2019 17:00
How much energy is supplied to each coulomb of charge that flows through a 12-v battery?
Answers: 1
You know the right answer?
MODERN PHYSICS
A photon emitted from an excited hydrogen atom has an energy of 3.02 elec...
A photon emitted from an excited hydrogen atom has an energy of 3.02 elec...
Questions

Chemistry, 28.04.2021 01:00

Health, 28.04.2021 01:00


Mathematics, 28.04.2021 01:00

Mathematics, 28.04.2021 01:00



Mathematics, 28.04.2021 01:00


Chemistry, 28.04.2021 01:00



Mathematics, 28.04.2021 01:00



Mathematics, 28.04.2021 01:00

Biology, 28.04.2021 01:00


Mathematics, 28.04.2021 01:00

Mathematics, 28.04.2021 01:00