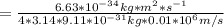German physicist Werner Heisenberg related the uncertainty of an object's position (Δx) to the uncertainty in its velocity (Δv)Δx≥h4πmΔvwhere h is Planck's constant and m is the mass of the object. The mass of an electron is 9.11×10−31 kg. What is the uncertainty in the position of an electron moving at 3.00×106 m/s with an uncertainty of Δv=0.01×106 m/s?Δ

Answers: 3


Another question on Physics



Physics, 22.06.2019 06:20
Oxygen undergoes an isentropic expansion from 120 degree c and 280 kpa absolute to 140 kpa absolute. determine the final temperature of the oxygen and the amount of work per kg of o_2 produced in the process if it is adiabatic.
Answers: 1

Physics, 22.06.2019 11:00
1. jay fills a wagon with sand (about 20 kg) and pulls it with a rope 30 m along the beach. he holds the rope 25° above the horizontal. the rope exerts a 20-n tension force on the wagon. how much work does the rope do on the wagon?
Answers: 1
You know the right answer?
German physicist Werner Heisenberg related the uncertainty of an object's position (Δx) to the uncer...
Questions

Mathematics, 17.07.2019 03:30

History, 17.07.2019 03:30


Mathematics, 17.07.2019 03:30

Mathematics, 17.07.2019 03:30

Mathematics, 17.07.2019 03:30

Mathematics, 17.07.2019 03:30



Mathematics, 17.07.2019 03:30

History, 17.07.2019 03:30




Social Studies, 17.07.2019 03:40


Social Studies, 17.07.2019 03:40

Biology, 17.07.2019 03:40