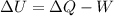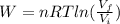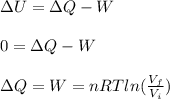An ideal monatomic gas at temperature T is held in a container. If the gas is compressed isothermally, that is at constant temperature, from a volume of Vi to Vf ,
a) What is the change in the (internal) energy of the gas?
b) How much work has been done on the gas?
c) Has heat been transferred into or out of the gas during the process? If so, how much?
d) Show that the 1st law of thermodynamics is satisfied.

Answers: 3


Another question on Physics

Physics, 22.06.2019 01:10
Suppose that two tanks, 1 and 2, each with a large opening at the top, contain different liquids. a small hole is made in the side of each tank at the same depth 1.57 m below the liquid surface, but the hole in tank 2 has 2.49 times the cross-sectional area of the hole in tank 1. (a) what is the ratio ρ1/ρ2 of the densities of the liquids if the mass flow rate is the same for the two holes? (b) what is the ratio rv1/rv2 of the volume flow rates from the two tanks? (c) at one instant, the liquid in tank 1 is 14.9 cm above the hole. if the tanks are to have equal volume flow rates, what height above the hole must the liquid in tank 2 be just then?
Answers: 3


Physics, 22.06.2019 06:30
=force × distance a. work b. velocity c. pressure d. momentum
Answers: 1

Physics, 22.06.2019 09:50
If an athlete runs once around a track, back to the starting line, her average velocity is zero. true or false?
Answers: 3
You know the right answer?
An ideal monatomic gas at temperature T is held in a container. If the gas is compressed isothermall...
Questions

English, 21.10.2019 23:00


Chemistry, 21.10.2019 23:00

Geography, 21.10.2019 23:00

Mathematics, 21.10.2019 23:00


Mathematics, 21.10.2019 23:00

Mathematics, 21.10.2019 23:00



Social Studies, 21.10.2019 23:00

History, 21.10.2019 23:00



Computers and Technology, 21.10.2019 23:00