
Physics, 05.05.2020 05:31 kutsevjulianna
Approximately 80% of the energy used by the body must be dissipated thermally. The mechanisms available to eliminate this energy are radiation, evaporation of sweat, evaporation from the lungs, conduction, and convection. In this question, we will focus on the evaporation of sweat alone, although all of these mechanisms are needed to survive. The latent heat of vaporization of sweat at body temperature (37 °C) is 2.42 x 10^6 J/kg and the specific heat of a body is approximately 3500 J/(kg*°C).
(A) To cool the body of a jogger of mass 90 kg by 1.8°C , how much sweat has to evaporate?
O 130 g
O 230 g
O 23 g
O 13 g

Answers: 1


Another question on Physics

Physics, 22.06.2019 15:30
The voltage applied across a given parallel-plate capacitor is doubled. how is the energy stored in the capacitor affected?
Answers: 2

Physics, 22.06.2019 22:00
Should we celebrate the voyages of zheng he mini q answer key
Answers: 3

Physics, 22.06.2019 23:00
A6.0-μf air-filled capacitor is connected across a 100-v voltage source. after the source fully charges the capacitor, the capacitor is immersed in transformer oil (of dielectric constant 4.5). how much additional charge flows from the voltage source, which remained connected during the process?
Answers: 1

You know the right answer?
Approximately 80% of the energy used by the body must be dissipated thermally. The mechanisms availa...
Questions






Social Studies, 01.12.2019 08:31

Physics, 01.12.2019 08:31

Mathematics, 01.12.2019 08:31

Advanced Placement (AP), 01.12.2019 08:31


Advanced Placement (AP), 01.12.2019 08:31




Advanced Placement (AP), 01.12.2019 08:31




Mathematics, 01.12.2019 08:31

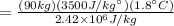
 ΔT
ΔT



