
Physics, 06.05.2020 03:01 daisyramirez2057
A 6.00 kg piece of solid copper metal at an initial temperature T is placed with 2.00 kg of ice that is initially at -10.0 ∘C. The ice is in an insulated container of negligible mass and no heat is exchanged with the surroundings. After thermal equilibrium is reached, there is 0.50 kg of ice and 1.50 kg of liquid water.
Required:
What was the initial temperature of the piece of copper?

Answers: 1


Another question on Physics

Physics, 21.06.2019 15:50
The harmonic motion of a particle is given by f(t) = 2 cos(3t) + 3 sin(2t), 0 ? t ? 8. (a) when is the position function decreasing? (round you
Answers: 1

Physics, 21.06.2019 21:30
One day, after pulling down your window shade, you notice that sunlight is passing through a pinhole in the shade and making a small patch of light on the far wall. you see that the patch of light seems to be a circular diffraction pattern. it appears that the central maximum is about 1 cm across and you estimate that the distance from the window shade to the wall is about 3 m. what is (a) the average wavelength of sunlight? (b) the diameter of the pinhole?
Answers: 3

Physics, 22.06.2019 10:00
What is the temperature in degrees celsius of a substance with a tempature of 49k
Answers: 2

Physics, 22.06.2019 14:30
What distance does a car travel as its speed changes from 0 to 20 m/s in 17 s at constant acceleration?
Answers: 1
You know the right answer?
A 6.00 kg piece of solid copper metal at an initial temperature T is placed with 2.00 kg of ice that...
Questions




Biology, 29.07.2019 05:30


Mathematics, 29.07.2019 05:30


Physics, 29.07.2019 05:30

Spanish, 29.07.2019 05:30

History, 29.07.2019 05:30


Mathematics, 29.07.2019 05:30

Mathematics, 29.07.2019 05:30

Mathematics, 29.07.2019 05:30

Computers and Technology, 29.07.2019 05:30



Social Studies, 29.07.2019 05:30

History, 29.07.2019 05:30

History, 29.07.2019 05:30

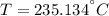
![(6000\,g) \cdot \left(0.385\,\frac{J}{g\cdot ^{\textdegree}C} \right)\cdot (T -0^{\textdegree}C ) = (2000\,g)\cdot \left(2.108\,\frac{J}{g\cdot ^{\textdegree}C} \right)\cdot [0^{\textdegree}C -(-10^{\textdegree}C)] + (1500\,g)\cdot \left(334\,\frac{J}{g} \right)](/tpl/images/0645/5025/aa197.png)


