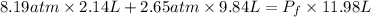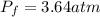Physics, 06.05.2020 07:35 darrenturner
The stopcock connecting a 2.14 L bulb containing oxygen gas at a pressure of 8.19 atm, and a 9.84 L bulb containing krypton gas at a pressure of 2.65 atm, is opened and the gases are allowed to mix. Assuming that the temperature remains constant, the final pressure in the system is atm.
what is the final pressure of the system in atm?

Answers: 1


Another question on Physics

Physics, 22.06.2019 17:00
If you wanted to move an electron from the positive to the negative terminal of the battery, how much work w would you need to do on the electron? enter your answer numerically in joules.
Answers: 1

Physics, 22.06.2019 21:50
Aforce of 8,480 n is applied to a cart to accelerate it at a rate of 26.5 m/s2. what is the mass of the cart?
Answers: 1

Physics, 22.06.2019 22:50
Which of the following statements correctly describes a transformer?
Answers: 3

Physics, 23.06.2019 03:30
An object is kept at 45 m high and how much time will it take to reach the ground
Answers: 2
You know the right answer?
The stopcock connecting a 2.14 L bulb containing oxygen gas at a pressure of 8.19 atm, and a 9.84 L...
Questions














Mathematics, 07.07.2020 22:01






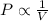
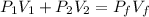
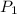 = first pressure = 8.19 atm
= first pressure = 8.19 atm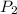 = second pressure = 2.65 atm
= second pressure = 2.65 atm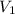 = first volume = 2.14 L
= first volume = 2.14 L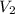 = second volume = 9.84 L
= second volume = 9.84 L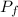 = final pressure = ?
= final pressure = ?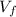 = final volume = 2.14 L + 9.84 L = 11.98 L
= final volume = 2.14 L + 9.84 L = 11.98 L