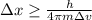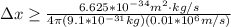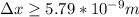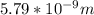German physicist Werner Heisenberg related the uncertainty of an object's position ( Δ x ) to the uncertainty in its velocity ( Δ v ) Δ x ≥ h 4 π m Δ v where h is Planck's constant and m is the mass of the object. The mass of an electron is 9.11 × 10 − 31 kg. What is the uncertainty in the position of an electron moving at 2.00 × 10 6 m/s with an uncertainty of Δ v = 0.01 × 10 6 m/s ?

Answers: 1


Another question on Physics

Physics, 21.06.2019 21:30
Look at the potential energy diagram for a chemical reaction. which statement correctly describes the energy changes that occur in the forward reaction?
Answers: 1

Physics, 22.06.2019 05:30
Iwill give 30 points to whoever answers ! what is the formula for actual mechanical advantage and ideal mechanical advantage?
Answers: 1

Physics, 22.06.2019 08:50
You are given a vector a = 125i and an unknown vector b that is perpendicular to a. the cross-product of these two vectors is a × b = 98k. what is the y-component of vector b?
Answers: 1

Physics, 22.06.2019 15:00
Sodium chloride, nacl, is formed when a sodium atom transfers its electron to a chlorine atom. the difference in charge between the two atoms creates a(n) attraction that bonds them together.
Answers: 1
You know the right answer?
German physicist Werner Heisenberg related the uncertainty of an object's position ( Δ x ) to the un...
Questions




Computers and Technology, 18.07.2020 01:01



Mathematics, 18.07.2020 01:01


Mathematics, 18.07.2020 01:01

Mathematics, 18.07.2020 01:01




Mathematics, 18.07.2020 01:01

Mathematics, 18.07.2020 01:01





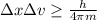
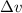 = Uncertainty in velocity of object
= Uncertainty in velocity of object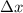 = Uncertainty in position of object
= Uncertainty in position of object