
Physics, 18.04.2020 20:43 Angelanova69134
Atmospheric pressure at the top of Mount Everest is about 150 mmHg that’s why climbers always bring oxygen tanks if a climber carries a 15.0 L tank with a pressure of 35, 000 mmHg what volume of the gas occupy if it is released at the top of Mount Everest
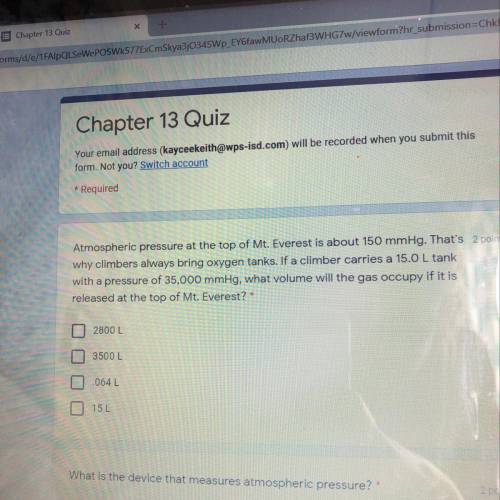

Answers: 2


Another question on Physics

Physics, 22.06.2019 13:10
A0.750 kg aluminum pan is removed from the stove and plunged into a sink filled with 10.0 kg of water at 293 k. the water temperature quickly rises to 297 k. what was the initial temperature of the aluminum pan? the specific heat of aluminum is ca = 900 j/(kgk) and water is cw = 4190 j/(kgk)
Answers: 3

Physics, 22.06.2019 16:30
Select all the correct answers. which three statements about electromagnetic radiation are true?
Answers: 3

Physics, 22.06.2019 18:00
Consider an ideal gas at 27.0 degrees celsius and 1.00 atmosphere pressure. imagine the molecules to be uniformly spaced, with each molecule at the center of a small cube. what is the length l of an edge of each small cube if adjacent cubes touch but don't overlap?
Answers: 2

Physics, 23.06.2019 03:30
The applied force of 3 washers will increase the applied force on the car to n.
Answers: 1
You know the right answer?
Atmospheric pressure at the top of Mount Everest is about 150 mmHg that’s why climbers always bring...
Questions


Mathematics, 19.07.2021 15:50

History, 19.07.2021 15:50



English, 19.07.2021 15:50







Business, 19.07.2021 15:50



Computers and Technology, 19.07.2021 15:50



Mathematics, 19.07.2021 15:50

Physics, 19.07.2021 15:50



