
1. in a single atom, no more than 2 electrons can occupy a single orbital? a. true b. false
2. the maximum number of electrons allowed in a p sublevel of the 3rd principal level is?
a. 1 b.6 c. 2 d. 8 e. 3
3. a neutral atom has a ground state electronic configuration of 1s^2 2s^2. which of the following statements concerning this atom is/are correct? a. the atom has a total of two orbitals occupied. b. all of the above. c. the atom has no unpaired electrons. d. the atom contains 4 protons. e. the element has atomic number 4.

Answers: 3


Another question on Physics

Physics, 21.06.2019 22:00
What is the fate of the electrons that interact with a specimen in an electron microscope?
Answers: 1

Physics, 22.06.2019 01:20
An engineer designs a roller coaster so that a car travels horizontally for 152 ft, then climbs 137 ft at an angle of 34.0° above the horizontal. it then moves 137 ft at an angle of 48.0° below the horizontal. if we take the initial horizontal motion of the car to be along the +x-axis, what is the car's displacement? (give the magnitude of your answer, in ft, to at least four significant figures and give the direction of your answer in degrees counterclockwise from the +x-axis.)
Answers: 1

Physics, 22.06.2019 19:00
The law of reflection states that the angle of reflection is equal to the angle of
Answers: 1

Physics, 23.06.2019 03:00
Does science support the idea that humans have influenced global warming? provide specific examples that show how two knowledgeable individuals with differing opinions might argue.
Answers: 2
You know the right answer?
1. in a single atom, no more than 2 electrons can occupy a single orbital? a. true b. false
...
...
Questions


Chemistry, 15.11.2021 01:00



Mathematics, 15.11.2021 01:00

Engineering, 15.11.2021 01:00

English, 15.11.2021 01:00


Mathematics, 15.11.2021 01:00


Mathematics, 15.11.2021 01:00


Mathematics, 15.11.2021 01:00

Chemistry, 15.11.2021 01:00



Health, 15.11.2021 01:00



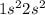 .
.


