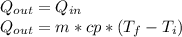reaches an equilibrium temperature of 31.1°C.

Physics, 17.04.2020 06:47 johnisawesome999
A jar of tea is placed in sunlight until it
reaches an equilibrium temperature of 31.1°C.
In an attempt to cool the liquid, which has a
mass of 177 g. 110 g of ice at 0.0°C is added.
At the time at which the temperature of the
tea is 29.1°C, find the mass of the remaining ice in the jar. The specific heat of water is 4186 J/kg.°C. Assume the specific heat capacity of the tea to be that of pure liquid water. Answer in units of g.

Answers: 2


Another question on Physics

Physics, 22.06.2019 14:00
Often called simply "velocity," this is the velocity of an object at a particular moment in time.
Answers: 1

Physics, 22.06.2019 16:00
The electric potential v is constant everywhere within a certain region of space. which statement below is true? the choices are: the electric field is also constant (but not zero) within the region. a charged particle placed within the region will experience an electric force. the electric field is zero everywhere within the region. the electric field varies from place to place within the region.
Answers: 2

Physics, 22.06.2019 17:40
Emmy kicks a soccer ball up at an angle of 45° over a level field. she watches the ball's trajectory and notices that it lands, two seconds after being kicked, about 20 m away to the north. assume that air resistance is negligible, and plot the horizontal and vertical components of the ball's velocity as a function of time. consider only the time that the ball is in the air, after being kicked but before landing. take "north" and "up" as the positive ‑ and ‑directions, respectively, and use ≈10 m/s2 for the downward acceleration due to gravity.
Answers: 2

Physics, 22.06.2019 19:40
Uranium has two naturally occurring isotopes. 238u has a natural abundance of 99.3% and 235u has an abundance of 0.7%. it is the rarer 235u that is needed for nuclear reactors. the isotopes are separated by forming uranium hexafluoride uf6, which is a gas, then allowing it to diffuse through a series of porous membranes. 235uf6 has a slightly larger rms speed than 238uf6 and diffuses slightly faster. many repetitions of this procedure gradually separate the two isotopes. what is the ratio of the rms speed of 235uf6 to that of 238uf6? express your answer to five significant figures.
Answers: 3
You know the right answer?
A jar of tea is placed in sunlight until it
reaches an equilibrium temperature of 31.1°C.
reaches an equilibrium temperature of 31.1°C.
Questions

History, 07.12.2021 21:50

English, 07.12.2021 21:50

Mathematics, 07.12.2021 21:50


Mathematics, 07.12.2021 21:50


SAT, 07.12.2021 21:50

SAT, 07.12.2021 21:50

History, 07.12.2021 21:50



Business, 07.12.2021 21:50

Mathematics, 07.12.2021 21:50

Mathematics, 07.12.2021 21:50




Mathematics, 07.12.2021 21:50

English, 07.12.2021 21:50