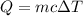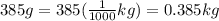Physics, 15.04.2020 03:26 atkinsonsinbraz
A 385 g glass coffee cup is at a room temperature of 20◦C. It is then plunged into hot dishwater at 78◦C. If the temperature of the cup reaches that of the dishwater, how much heat does the cup absorb? Assume the mass of the dishwater is large enough so its temperature doesn’t change appreciably and the specific heat of glass is 840 J/kg · ◦ C. Answer in units of J.

Answers: 2


Another question on Physics

Physics, 22.06.2019 16:20
How does a circuit breaker protect a refrigerator? a. when the current is too high, a metal strip in the fuse melts and opens the circuit. b. when the resistance is too high , a re-settable which opens a circuit c. when the current is too high , a re-settable switch opens the circuit d. when the resistance is too high a metal strip in the fuse melts and opens the circuit
Answers: 2

Physics, 22.06.2019 17:40
Scientists follow specific processes in order to determine valid explanations and conclusions from observations. david observed strange lights in the sky above his home outside of roswell, new mexico. the only explanation that makes sense to him is that there are ufos in the area. what should david do next to verify his explanation?
Answers: 1


Physics, 23.06.2019 09:30
How many milliliters of water at 23 °c with a density of 1.00 g/ml must be mixed with 180 ml (about 6 oz) of coffee at 95 °c so that the resulting combination will have a temperature of 60 °c? assume that coffee and water have the same density and the same specific heat. how much will the temperature of a cup (180 g) of coffee at 95 °c be reduced when a 45 g silver spoon (specific heat 0.24 j/g °c) at 25 °c is placed in the coffee and the two are allowed to reach the same temperature? assume that the coffee has the same density and specific heat as water. a 45-g aluminum spoon (specific heat 0.88 j/g °c) at 24 °c is placed in 180 ml (180 g) of coffee at 85 °c and the temperature of the two become equal. (a) what is the final temperature when the two become equal? assume that coffee has the same specific heat as water. (b) the first time a student solved this problem she got an answer of 88 °c. explain why this is clearly an incorrect answer.
Answers: 1
You know the right answer?
A 385 g glass coffee cup is at a room temperature of 20◦C. It is then plunged into hot dishwater at...
Questions


Mathematics, 20.11.2021 08:00




Social Studies, 20.11.2021 08:00


Mathematics, 20.11.2021 08:00


Social Studies, 20.11.2021 08:00

Arts, 20.11.2021 08:00

Social Studies, 20.11.2021 08:00

Mathematics, 20.11.2021 08:00

History, 20.11.2021 08:00

World Languages, 20.11.2021 08:00

Mathematics, 20.11.2021 08:00


Mathematics, 20.11.2021 08:00

Physics, 20.11.2021 08:00