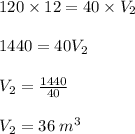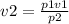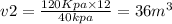Answers: 2


Another question on Physics

Physics, 21.06.2019 20:30
Protons and neutrons are found within the nucleus of an atom
Answers: 2

Physics, 22.06.2019 09:30
On a day when the barometer reads 75.23 cm, a reaction vessel holds 250 ml of ideal gas at 20 celsius. an oil manometer ( ρ= 810 kg/m^3) reads the pressure in the vessel to be 41 cm of oil and below atmospheric pressure. what volume will the gas occupy under s.t.p.?
Answers: 2

Physics, 22.06.2019 14:00
How much energy must a refrigerator absorb from 225 g of water so that the temperature of the water will drop from 35°c to 5°c
Answers: 3

You know the right answer?
A helium balloon containing 12m³ of gas at a pressure of 120kPa is released into the air. Assuming t...
Questions




Mathematics, 05.03.2020 16:10






Mathematics, 05.03.2020 16:12



Biology, 05.03.2020 16:13



English, 05.03.2020 16:13

History, 05.03.2020 16:14



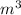 Initial pressure = 120 kPaFinal pressure = 40 kPa
Initial pressure = 120 kPaFinal pressure = 40 kPa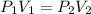
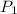 is the original (initial) pressure.
is the original (initial) pressure.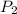 is the final pressure.
is the final pressure.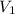 is the original (initial) volume.
is the original (initial) volume.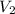 is the final volume.
is the final volume.