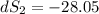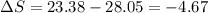One container holds 0.10 kg of water at 75 ∘C and is warmed to 95 ∘C by heating from contact with the other container. The other container, also holding 0.10 kg of water, cools from 35 ∘C to 15 ∘C. Specific heat of water is 4180 J/kg⋅∘C. Estimate the total change in entropy of two containers of water using the actual temperatures to determine the heat transferred to each container and the average temperatures to determine the change in entropy. Is this energy transfer process allowed by the first law of thermodynamics? Yes or No. is this energy transfer process allowed by the second law of thermodynamics? Yes or No

Answers: 1


Another question on Physics

Physics, 22.06.2019 02:00
An object has a weight of 500 on earth what is the mass of this object
Answers: 1

Physics, 22.06.2019 16:00
The electric potential v is constant everywhere within a certain region of space. which statement below is true? the choices are: the electric field is also constant (but not zero) within the region. a charged particle placed within the region will experience an electric force. the electric field is zero everywhere within the region. the electric field varies from place to place within the region.
Answers: 2


Physics, 23.06.2019 05:00
Two cans on a sunny window one black and the other white construct and explanation of the effect of sun will have on both cans over a two hour period?
Answers: 1
You know the right answer?
One container holds 0.10 kg of water at 75 ∘C and is warmed to 95 ∘C by heating from contact with th...
Questions

Chemistry, 28.10.2020 17:30







Mathematics, 28.10.2020 17:30

Mathematics, 28.10.2020 17:30





Computers and Technology, 28.10.2020 17:30


History, 28.10.2020 17:30

Mathematics, 28.10.2020 17:30



History, 28.10.2020 17:30

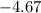
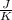
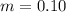 Kg
Kg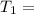 75°C
75°C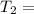 95°C
95°C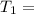 35°C
35°C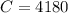
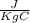
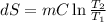
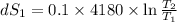
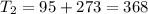 K,
K, 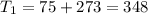 K
K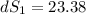
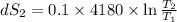
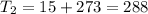 K,
K, 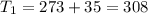 K
K