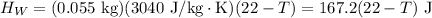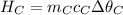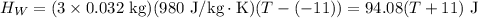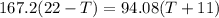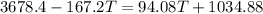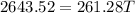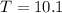Physics, 25.03.2020 17:59 kandrews6221
If you pour whisky over ice, the ice will cool the drink, but it will also dilute it. A solution is to use whisky stones. Suppose Ernest pours 55.0 g of whisky at 22 ∘C room temperature, and then adds three whisky stones to cool it. Each stone is a 32.0 g soapstone cube that is stored in the freezer at -11 ∘C. The specific heat of soapstone is 980 J/kg⋅K; the specific heat of whisky is 3400 J/kg⋅K. What is the final temperature of the whisky?

Answers: 1


Another question on Physics

Physics, 22.06.2019 06:00
What will a positive and a negative charge do if they are separated from each other?
Answers: 3

Physics, 22.06.2019 06:10
Which transition by an electron will release the greatest amount of energy? oa ob oc od
Answers: 2

Physics, 22.06.2019 11:10
Rank the automobiles based on the magnitude of the force needed to stop them, from largest to smallest.2000 kg car going 5m/s500 kg car going 20 m/s1000 kg car going20m/s500 kg car going10m/s1000 kg car going10/s4000 kg car going 5m/s 0 0 603
Answers: 3

You know the right answer?
If you pour whisky over ice, the ice will cool the drink, but it will also dilute it. A solution is...
Questions

Mathematics, 06.05.2020 02:19

Mathematics, 06.05.2020 02:19




Mathematics, 06.05.2020 02:19


Mathematics, 06.05.2020 02:19









Mathematics, 06.05.2020 02:19



Mathematics, 06.05.2020 02:19

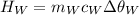
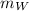 is the mass of the whisky (in kg),
is the mass of the whisky (in kg), 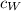 is the specific heat of whisky (J/kg·K) and
is the specific heat of whisky (J/kg·K) and 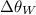 is the change in temperature of the whisky (in °C or K).
is the change in temperature of the whisky (in °C or K).