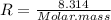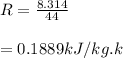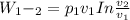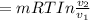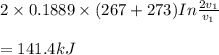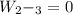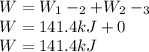Mass of 4 kilograms of carbon dioxide (CO2) in a piston-cylinder assembly undergoes two processes in series from an initial state where p1 = 0.5 MPa, T1 = 267°C: Process 1–2: Constant-temperature expansion until the volume is twice the initial volume. Process 2–3: Constant-volume heating until the pressure is again 0.5 MPa. Assuming ideal gas behavior, determine the overall work, in kJ.

Answers: 3


Another question on Physics

Physics, 21.06.2019 16:00
When fat comes in contact with sodium hydroxide, it produces soap and glycerin. determine whether this is a physical change or a chemical change. explain your answer
Answers: 2


Physics, 22.06.2019 11:50
Find the dimensions and area of the largest rectangle that can be inscribed in the upper half of the ellipse. (give your answers in terms of a and b. enter the dimensions as a comma-separated list.)
Answers: 2

Physics, 22.06.2019 17:30
Does heating a metal wire increase or decrease its electrical resistance? why?
Answers: 1
You know the right answer?
Mass of 4 kilograms of carbon dioxide (CO2) in a piston-cylinder assembly undergoes two processes in...
Questions



Mathematics, 30.11.2020 21:40





Computers and Technology, 30.11.2020 21:40






Physics, 30.11.2020 21:40





Mathematics, 30.11.2020 21:40

History, 30.11.2020 21:40