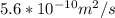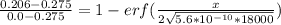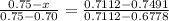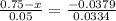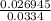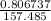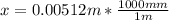An iron-carbon alloy initially containing 0.275 wt% C is exposed to an oxygen-rich and virtually carbon-free atmosphere at 1110°C. Under these circumstances the carbon diffuses from the alloy and reacts at the surface with the oxygen in the atmosphere; that is, the carbon concentration at the surface position is maintained essentially at 0.0 wt% C. At what position will the carbon concentration be 0.206 wt% after a 5 h treatment? The value of D at 1110°C is 5.6 × 10-10 m2/s.

Answers: 1


Another question on Physics

Physics, 21.06.2019 15:30
What defines the mass number of an isotope? a. the sum of the neutrons and protons b. the sum of the neutrons and electrons c. the number of neutrons d. the number of protons
Answers: 1

Physics, 22.06.2019 02:30
Acup of coffee sits on the dashboard of an automobile. the car goes around a sharp curve. even though you hold the cup still, coffee still splashes out. this can best be explained due to a) density. b) friction. c) gravity. d) inertia.
Answers: 2

Physics, 22.06.2019 06:30
How much force was applied to an object if was moved 2 meters and the work done on the object was 8 joules? a. 0.25 n b. 4 n c. 6 n d. 16 n
Answers: 1

Physics, 22.06.2019 12:00
An architect plans to use solar energy to heat the next house he designs. what principle of absorption and infrared energy can be applied to the design of the new house? how could she apply to those principals?
Answers: 2
You know the right answer?
An iron-carbon alloy initially containing 0.275 wt% C is exposed to an oxygen-rich and virtually car...
Questions



English, 21.06.2021 19:20

Mathematics, 21.06.2021 19:20

English, 21.06.2021 19:20

Mathematics, 21.06.2021 19:20

Computers and Technology, 21.06.2021 19:20

Physics, 21.06.2021 19:20

Mathematics, 21.06.2021 19:20

Mathematics, 21.06.2021 19:20

Biology, 21.06.2021 19:20



English, 21.06.2021 19:20

Mathematics, 21.06.2021 19:20


Mathematics, 21.06.2021 19:20


Computers and Technology, 21.06.2021 19:20

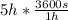
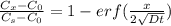
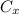 = concentration at depth = 0.206 wt%
= concentration at depth = 0.206 wt%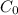 = initial concentration = 0.275 wt%
= initial concentration = 0.275 wt%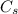 = concentration at the surface position = 0.0 wt%
= concentration at the surface position = 0.0 wt%