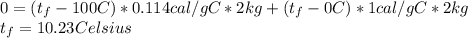Physics, 17.03.2020 04:23 lizbethmillanvazquez
A 2-kg piece of iron is heated to 100°C, and then submerged in 2 kg of water initially at 0°C. The iron cools and the water warms until they are at the same temperature (in thermal equilibrium). Assuming there is no other transfer of heat involved, is the final temperature closer to 0°C, 50°C, or 100°C?

Answers: 1


Another question on Physics

Physics, 22.06.2019 00:30
Using the work energy theorem. what is the velocity of a 850kg car after starting at rest when 13,000j of work is done to it.
Answers: 1

Physics, 22.06.2019 05:30
Agas expands from an initial volume of 0.040 m^3 and an initial pressure of 210 kpa to a final volume of 0.065 m^3 while its temperature is kept constant. how much work is done by the system?
Answers: 1

Physics, 22.06.2019 07:10
Polly is pushing a box across the floor with a force of 30 n. the force of gravity is –8 n, and the normal force is 8 n. which value could describe the force of friction if polly could not move the box?
Answers: 2

Physics, 22.06.2019 11:30
Considering only the earth's rotation, determine how much later the asteroid would have had to arrive to put the explosion above helsinki at longitude 25˚ e? this would have obliterated the city.
Answers: 1
You know the right answer?
A 2-kg piece of iron is heated to 100°C, and then submerged in 2 kg of water initially at 0°C. The i...
Questions

Mathematics, 02.03.2020 17:34



Mathematics, 02.03.2020 17:34


Biology, 02.03.2020 17:34




Mathematics, 02.03.2020 17:34


History, 02.03.2020 17:34







Mathematics, 02.03.2020 17:34

Biology, 02.03.2020 17:34