Physics, 13.03.2020 03:59 sassy11111515
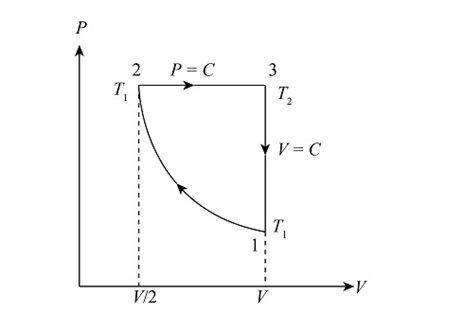
A heat engine using a diatomic ideal gas goes through the following closed cycle: ∙ Isothermal compression until the volume is halved. ∙ Isobaric expansion until the volume is restored to its initial value. ∙ Isochoric cooling until the pressure is restored to its initial value.

Answers: 3


Another question on Physics

Physics, 21.06.2019 23:30
The pressure, volume, and temperature of a mole of an ideal gas are related by the equation pv = 8.31t, where p is measured in kilopascals, v in liters, and t in kelvins. use differentials to find the approximate change in the pressure if the volume increases from 14 l to 14.6 l and the temperature decreases from 375 k to 370 k. (round the answer to two decimal places.)
Answers: 3

Physics, 22.06.2019 03:30
Two polarizers are oriented at 24.0∘ to one another. light polarized at a 12.0-degree angle to each polarizer passes through both. what is the transmitted intensity (%)?
Answers: 2

Physics, 22.06.2019 15:30
What is a subatomic particle with a negative charge and very little mass?
Answers: 1

Physics, 22.06.2019 16:40
Panel bc in fig. p2.76 is semi-circular, with the 3 meter radius and horizontal straight edge through point b. compute (a) the hydrostatic force of the water on the panel, (b) its center of pressure, and (c) the moment of this force about point b. assume atmospheric pressure on the dry side of the panel
Answers: 3
You know the right answer?
A heat engine using a diatomic ideal gas goes through the following closed cycle: ∙ Isothermal compr...
Questions


Mathematics, 21.01.2021 03:20



Mathematics, 21.01.2021 03:20

Mathematics, 21.01.2021 03:20

Mathematics, 21.01.2021 03:20


History, 21.01.2021 03:20


Chemistry, 21.01.2021 03:20


English, 21.01.2021 03:20

History, 21.01.2021 03:20

Mathematics, 21.01.2021 03:20




Biology, 21.01.2021 03:20