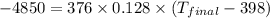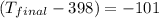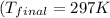Physics, 10.03.2020 03:03 meganwintergirl
Determine the final temperature of a gold nugget (mass = 376 g) that starts at 398 K and loses 4.85 kJ of heat to a snowbank when it is lost. The specific heat capacity of gold is 0.128 J/g°C.

Answers: 1


Another question on Physics


Physics, 21.06.2019 22:10
Agas is contained in a vertical, frictionless piston–cylinder device. the piston has a mass of 3.2 kg and a cross-sectional area of 35 cm2. a compressed spring above the piston exerts a force of 190 n on the piston. if the atmospheric pressure is 95 kpa, determine the pressure inside the cylinder.
Answers: 3

Physics, 22.06.2019 04:00
Ametal ball with a mass of 0.028 kg is dropped from rest at a height of 1.0 meters above the ground. assuming the energy in the ball is conserved, how much kinetic energy will the ball have when it is 0.5 meters above the ground?
Answers: 1

You know the right answer?
Determine the final temperature of a gold nugget (mass = 376 g) that starts at 398 K and loses 4.85...
Questions

Mathematics, 05.05.2020 07:24





Mathematics, 05.05.2020 07:24

Mathematics, 05.05.2020 07:24

Social Studies, 05.05.2020 07:24




Mathematics, 05.05.2020 07:24




Biology, 05.05.2020 07:24



Mathematics, 05.05.2020 07:24

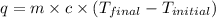
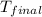 = final temperature of metal = ?
= final temperature of metal = ? 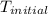 = initial temperature of metal = 398 K
= initial temperature of metal = 398 K