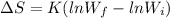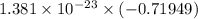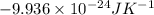Physics, 10.03.2020 02:12 ashiteru123
A gaseous system undergoes a change in temperature and volume. What is the entropy change for a particle in this system if the final number of microstates is 0.559 times that of the initial number of microstates?

Answers: 3


Another question on Physics

Physics, 21.06.2019 20:30
Air enters a compressor operating at steady state at 1.05 bar, 300 k, with a volumetric flow rate of 93 m3/min and exits at 12 bar, 400 k. heat transfer occurs at a rate of 15.5 kw from the compressor to its surroundings. assuming the ideal gas model for air neglecting kinetic potential energy effects, determine the power in put in kw.
Answers: 2

Physics, 22.06.2019 13:30
Global warming will produce rising sea levels partly due to melting ice caps but also due to the expansion of water as average ocean temperatures rise. to get some idea of the size of this effect, calculate the change in length of a column of water 1.00 km high for a temperature increase of 1.00ºc. note that this calculation is only approximate because ocean warming is not uniform with depth. (answer in ×10^{-3} −3 m)
Answers: 1


Physics, 23.06.2019 02:00
How do you count the total number of atoms present on one side of a chemical equation?
Answers: 1
You know the right answer?
A gaseous system undergoes a change in temperature and volume. What is the entropy change for a part...
Questions






History, 28.03.2020 21:10


Engineering, 28.03.2020 21:10




English, 28.03.2020 21:10


Social Studies, 28.03.2020 21:11

Mathematics, 28.03.2020 21:11


English, 28.03.2020 21:11


English, 28.03.2020 21:11

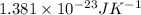
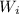 = 1 then final
= 1 then final  = 0.487
= 0.487