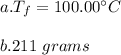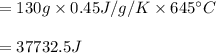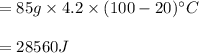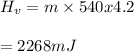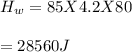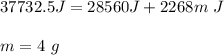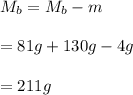You cool a 130.0 g slug of red-hot iron (temperature 745 ∘C) by dropping it into an insulated cup of negligible mass containing 85.0 g of water at 20.0 ∘C. Assume no heat exchange with the surroundings. How do you do this?
Part A What is the final temperature of the water?
Part B What is the final mass of the iron and the remaining water?

Answers: 1


Another question on Physics

Physics, 21.06.2019 18:10
Amass has an acceleration of 10- 2t. what is its velocity and displacement if it started at x- 4m and the initial velocity was 8m/s? at what time does it have 0 velocity? graph all graphs.
Answers: 2

Physics, 21.06.2019 21:30
Look at the potential energy diagram for a chemical reaction. which statement correctly describes the energy changes that occur in the forward reaction?
Answers: 2

Physics, 22.06.2019 00:40
The rigid beam is supported by the three suspender bars. bars ab and ef are made of aluminum and bar cd is made of steel. if each bar has a cross-sectional area of 450 mm2, determine the maximum value of p if the allowable stress is (σallow)st = 200 mpa for the steel and ( σallow)al = 150 mpa for the aluminum. est = 200 gpa and eal = 70 gpa.
Answers: 1

Physics, 22.06.2019 04:30
Determine el momento angular de un rueda de 3000g moviendose a 10m/s con un radio de giro de 400cm
Answers: 2
You know the right answer?
You cool a 130.0 g slug of red-hot iron (temperature 745 ∘C) by dropping it into an insulated cup of...
Questions

Mathematics, 11.10.2020 06:01

Mathematics, 11.10.2020 06:01

Biology, 11.10.2020 06:01


English, 11.10.2020 06:01

Mathematics, 11.10.2020 06:01

Computers and Technology, 11.10.2020 06:01


Mathematics, 11.10.2020 06:01

Mathematics, 11.10.2020 06:01



Mathematics, 11.10.2020 06:01


Health, 11.10.2020 06:01

History, 11.10.2020 06:01


Physics, 11.10.2020 06:01


Biology, 11.10.2020 06:01