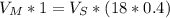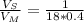Physics, 04.03.2020 23:33 hannahelisabeth19
A major artery with a cross-sectional area of 1.00cm2 branches into 18 smaller arteries, each with an average cross-sectional area of 0.400cm2. By what factor is the average velocity of the blood reduced when it passes into these branches

Answers: 2


Another question on Physics

Physics, 21.06.2019 20:20
When many atoms are split in a chain reaction, a large explosion occurs. this is an example of what type of energy conservation
Answers: 2

Physics, 22.06.2019 00:30
Part f - example: finding two forces (part i) two dimensional dynamics often involves solving for two unknown quantities in two separate equations describing the total force. the block in (figure 1) has a mass m=10kg and is being pulled by a force f on a table with coefficient of static friction îľs=0.3. four forces act on it: the applied force f (directed î¸=30â above the horizontal). the force of gravity fg=mg (directly down, where g=9.8m/s2). the normal force n (directly up). the force of static friction fs (directly left, opposing any potential motion). if we want to find the size of the force necessary to just barely overcome static friction (in which case fs=îľsn), we use the condition that the sum of the forces in both directions must be 0. using some basic trigonometry, we can write this condition out for the forces in both the horizontal and vertical directions, respectively, as: fcosî¸â’îľsn=0 fsinî¸+nâ’mg=0 in order to find the magnitude of force f, we have to solve a system of two equations with both f and the normal force n unknown. use the methods we have learned to find an expression for f in terms of m, g, î¸, and îľs (no n).
Answers: 2

Physics, 22.06.2019 05:50
Acylinder with a movable piston contains 11.7 moles of a monatomic ideal gas at a pressure of 1.32×10^5 pa. the gas is initially at a temperature of 300 k. an electric heater adds 43200 j of energy into the gas while the piston moves in such a way that the pressure remains constant. cp=20.79 j k^−1 mol^−1 for a monatomic ideal gas, and that the number of gas molecules is equal to avogadro's number (6.022×10^23) times the number of moles of the gas. (a) what is the temperature of the gas after the energy is added? (b) what is the change in volume of the gas? (c) how much work is done by the gas during this process?
Answers: 3

Physics, 22.06.2019 18:30
Examples of states of consciousness include a. daydreaming b. dreaming during sleep c. hypnosis d. all of the above
Answers: 2
You know the right answer?
A major artery with a cross-sectional area of 1.00cm2 branches into 18 smaller arteries, each with a...
Questions

Mathematics, 27.10.2020 17:50




English, 27.10.2020 17:50






Physics, 27.10.2020 17:50


Mathematics, 27.10.2020 17:50


Mathematics, 27.10.2020 17:50

Chemistry, 27.10.2020 17:50


History, 27.10.2020 17:50

Mathematics, 27.10.2020 17:50