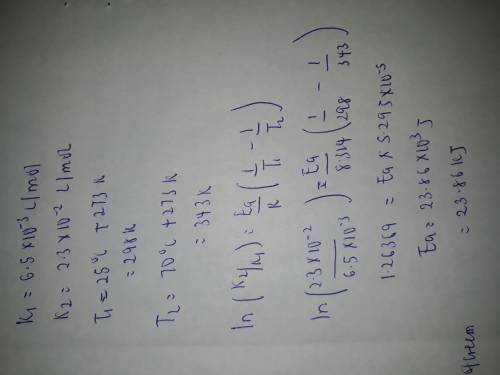Physics, 27.02.2020 03:19 Samonerob2002
A second-order reaction was observed. The reaction rate constant at 25 oC was found to be 6.50 x 10-3L/mol and at 70 oC it was found to be 2.30 x 10-2 L/mol. Calculate the activation energy of this reaction.

Answers: 2


Another question on Physics

Physics, 22.06.2019 05:40
The difference between a red shift and a blue shift has to do with wavelength frequency. t or f
Answers: 1

Physics, 22.06.2019 06:40
Use the right-hand rule for magnetic force to determine the charge on the moving particle. this is a charge.
Answers: 1

Physics, 23.06.2019 02:40
Check the following equation for dimensional homogeneity: ⅆ mv=∫t1t2(fcosθ)ⅆt where m is mass, v is velocity, f is force, θ is an angle, and t is time. check that each term in the equation has the following dimensions: [malbec] where you are to choose the coefficients a, b, c.
Answers: 2

Physics, 23.06.2019 19:30
1)the gradual change in the length of your shadow over the course of the day is caused 2)which of the following occurs when the sun is directly above earth’s equator? 3)in the northern hemisphere, the longest day occurs on the
Answers: 1
You know the right answer?
A second-order reaction was observed. The reaction rate constant at 25 oC was found to be 6.50 x 10-...
Questions

English, 27.06.2019 05:20

English, 27.06.2019 05:20


Mathematics, 27.06.2019 05:20



History, 27.06.2019 05:20

Mathematics, 27.06.2019 05:20

Spanish, 27.06.2019 05:20

Social Studies, 27.06.2019 05:20


Mathematics, 27.06.2019 05:20


Mathematics, 27.06.2019 05:20

Mathematics, 27.06.2019 05:20





Physics, 27.06.2019 05:20