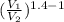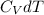Physics, 25.02.2020 04:34 hannahdrees0731
Two moles of carbon monoxide (CO) start at a pressure of 1.5 atm and a volume of 29 liters . The gas is then compressed adiabatically to 13 this volume. Assume that the gas may be treated as ideal.
(a) What is the change in the internal energy of the gas? Does the internal energy increase or decrease?
(b) Does the temperature of the gas increase or decrease during this process? Explain.

Answers: 3


Another question on Physics

Physics, 21.06.2019 15:00
If this plastic cup is heated to its melting point, what will eventually happen to all the particles in the plastic? a. the particles will begin to move enough that they slide past each other. b. bonds will form and the liquid will become a solid. c. the spaces between the particles will get smaller. d. the particles will change state and become a crystalline solid.
Answers: 2

Physics, 21.06.2019 19:20
Describe the path of the electric current through a circuit?
Answers: 1

Physics, 21.06.2019 20:20
An asteroid is discovered in a nearly circular orbit around the sun, with an orbital radius that is 2.47 times earth's. what is the asteroid's orbital period, its "year," in terms of earth years?
Answers: 1

Physics, 21.06.2019 20:30
Water at room temperature of 20.0°c is poured into an aluminum cylinder which has graduation markings etched on the inside. the reading in the graduations is 300.0 cc. the cylinder with the water in it is then immersed in a constant temperature bath at a temperature of 100°c. what is the reading for the level of water on the graduations of the cylinder after the water and the cylinder reach thermal equilibrium with the bath? the volume coefficient of expansion of water is 2.07 x 10^-4 k-1, and the linear coefficient of expansion of aluminum is 23.0 x 10^-6 k-1. a) 305.0 cc b) 304.0 cc c) 303.5 cc d) 303.3 cc e) 304.5 cc
Answers: 3
You know the right answer?
Two moles of carbon monoxide (CO) start at a pressure of 1.5 atm and a volume of 29 liters . The gas...
Questions

English, 05.05.2020 12:40


Mathematics, 05.05.2020 12:40

History, 05.05.2020 12:40

Biology, 05.05.2020 12:40

Business, 05.05.2020 12:40




Physics, 05.05.2020 12:40


Mathematics, 05.05.2020 12:40

History, 05.05.2020 12:40

Mathematics, 05.05.2020 12:40

History, 05.05.2020 12:40



Mathematics, 05.05.2020 12:40


Chemistry, 05.05.2020 12:40